Opinion statement
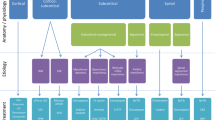
Treatment of myoclonus requires an understanding of the physiopathology of the condition. The first step in treatment is to determine if there is an epileptic component to the myoclonus and treat accordingly. Secondly, a review of medications (e.g., opiates) and comorbidities (e.g., hepatic or renal failure) is required to establish the possibility of iatrogenic and reversible conditions. Once those are eliminated, delineation between cortical, cortico-subcortical, subcortical, brainstem, and spinal generators can determine the first-line treatment. Cortical myoclonus can be treated with levetiracetam, valproic acid, and clonazepam as first-line agents. Phenytoin and carbamazepine may paradoxically worsen myoclonus. Subcortical and brainstem myoclonus can be treated with clonazepam as a first-line agent, but levetiracetam and valproic acid can be tried as well. l-5-Hydroxytryptophan and sodium oxybate are agents used for refractory cases. Spinal myoclonus does not respond to anti-epileptic drugs, and clonazepam is a first-line agent. Botulinum toxin treatment can be useful for focal cases of spinal myoclonus. The etiology of propriospinal myoclonus is controversial, and a functional etiology is suspected in most cases. Treatment can include clonazepam, levetiracetam, baclofen, valproate, carbamazepine, and zonisamide. Functional myoclonus requires multimodal and multidisciplinary treatment that may include psychotropic drugs and physical and occupational therapy. Close collaboration between neurologists and psychiatrists is required for effective treatment. Finally, deep brain stimulation targeting the globus pallidus pars-interna bilaterally has been used in myoclonus-dystonia when pharmacological treatments have been exhausted.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stanley Fahn JJ, Hallett M. Myoclonus: phenomenology, etiology, physiology, and treatment principles and practice of movement disorders. Edinburgh: Saunders; 2011. p. 447–64.
Ikeda A, Kakigi R, Funai N, et al. Cortical tremor: a variant of cortical reflex myoclonus. Neurology. 1990;40:1561–5.
Stanley Fahn JJ, Hallett M. Principles and practice of movement disorders. Edinburgh: Saunders; 2011.
Caviness JN, Brown P. Myoclonus: current concepts and recent advances. Lancet Neurol. 2004;3:598–607.
Espay AJ, Chen R. Myoclonus. Continuum (Minneap Minn). 2013;19:1264–86. This review focuses on the etiology, diagnosis, and electrophysiological evaluation of myoclonus as well as treatment.
Kurian M, Lalive PH, Dalmau JO, et al. Opsoclonus-myoclonus syndrome in anti-N-methyl-D-aspartate receptor encephalitis. Arch Neurol. 2010;67:118–21.
Turner MR, Irani SR, Leite MI, et al. Progressive encephalomyelitis with rigidity and myoclonus: glycine and NMDA receptor antibodies. Neurology. 2011;77:439–43.
Erlich R, Morrison C, Kim B, et al. ANNA-2: an antibody associated with paraneoplastic opsoclonus in a patient with large-cell carcinoma of the lung with neuroendocrine features—correlation of clinical improvement with tumor response. Cancer Investig. 2004;22:257–61.
Pike M. Opsoclonus-myoclonus syndrome. Handb Clin Neurol. 2013;112:1209–11.
Ganos C, Kassavetis P, Erro R, et al. The role of the cerebellum in the pathogenesis of cortical myoclonus. Mov Disord. 2014;29:437–43.
Terada K, Ikeda A, Van Ness PC, et al. Presence of bereitschaftspotential preceding psychogenic myoclonus: clinical application of jerk-locked back averaging. J Neurol Neurosurg Psychiatry. 1995;58:745–7.
Roze E, Bounolleau P, Ducreux D, et al. Propriospinal myoclonus revisited: clinical, neurophysiologic, and neuroradiologic findings. Neurology. 2009;72:1301–9. Article summarizes the clinical, electrophysiological, and neuroimaging features of propriosimal myoclonus. They report microstructual injuries that may explain some idiopathic cases.
van der Salm SM, Erro R, Cordivari C, et al. Propriospinal myoclonus: clinical reappraisal and review of literature. Neurology. 2014;83:1862–70.
van der Salm SM, Tijssen MA, Koelman JH, et al. The bereitschaftspotential in jerky movement disorders. J Neurol Neurosurg Psychiatry. 2012;83:1162–7.
Caviness JN. The clinical neurophysiology of myoclonus. In: Hallet M, editor. Handbook of clinical neurophysiology. Amsterdam: Elsevier; 2003.
Calandra-Buonaura G, Alessandria M, Liguori R, et al. Hypnic jerks: neurophysiological characterization of a new motor pattern. Sleep Med. 2014;15:725–7.
Caviness JN, Truong DD. Myoclonus. Handb Clin Neurol. 2011;100:399–420.
Angel MJ, Young GB. Metabolic encephalopathies. Neurol Clin. 2011;29:837–82.
Jimenez-Jimenez FJ, Puertas I, de Toledo-Heras M. Drug-induced myoclonus: frequency, mechanisms and management. CNS Drugs. 2004;18:93–104.
Deik AF, Shanker VL. A case of amiodarone-associated myoclonus responsive to levetiracetam. Can J Neurol Sci. 2012;39:680–1.
Brown P, Steiger MJ, Thompson PD, et al. Effectiveness of piracetam in cortical myoclonus. Mov Disord. 1993;8:63–8.
Obeso JA, Artieda J, Quinn N, et al. Piracetam in the treatment of different types of myoclonus. Clin Neuropharmacol. 1988;11:529–36.
Koskiniemi M, Van Vleymen B, Hakamies L, et al. Piracetam relieves symptoms in progressive myoclonus epilepsy: a multicentre, randomised, double blind, crossover study comparing the efficacy and safety of three dosages of oral piracetam with placebo. J Neurol Neurosurg Psychiatry. 1998;64:344–8.
Mbizvo GK, Dixon P, Hutton JL, et al. The adverse effects profile of levetiracetam in epilepsy: a more detailed look. Int J Neurosci. 2014;124:627–34.
Striano P, Manganelli F, Boccella P, et al. Levetiracetam in patients with cortical myoclonus: a clinical and electrophysiological study. Mov Disord. 2005;20:1610–4.
Frucht SJ, Bordelon Y, Houghton WH, et al. A pilot tolerability and efficacy trial of sodium oxybate in ethanol-responsive movement disorders. Mov Disord. 2005;20:1330–7.
Genton P, Gelisse P. Antimyoclonic effect of levetiracetam. Epileptic Disord. 2000;2:209–12.
Lexicomp Online®. Accessed October 17th, 2015.
Shin JH, Park JM, Kim AR, et al. Lance-Adams syndrome. Ann Rehabil Med. 2012;36:561–4.
Frucht SJ, Houghton WC, Bordelon Y, et al. A single-blind, open-label trial of sodium oxybate for myoclonus and essential tremor. Neurology. 2005;65:1967–9.
Menon MK. Antimyoclonic effect of sodium oxybate: clinical implications. JAMA. 1981;245:2495.
Arpesella R, Dallocchio C, Arbasino C, et al. A patient with intractable posthypoxic myoclonus (Lance-Adams syndrome) treated with sodium oxybate. Anaesth Intensive Care. 2009;37:314–8.
Magnussen I, Dupont E, Engbaek F, et al. Post-hypoxic intention myoclonus treated with 5-hydroxy-tryptophan and an extracerebral decarboxylase inhibitor. Acta Neurol Scand. 1978;57:289–94.
Van Woert MH, Sethy VH. Therapy of intention myoclonus with L-5-hydroxytryptophan and a peripheral decarboxylase inhibitor, MK 486. Neurology. 1975;25:135–40.
Deuschl G, Wilms H. Palatal tremor: the clinical spectrum and physiology of a rhythmic movement disorder. Adv Neurol. 2002;89:115–30.
Zadikoff C, Lang AE, Klein C. The ‘essentials’ of essential palatal tremor: a reappraisal of the nosology. Brain. 2006;129:832–40.
Stamelou M, Saifee TA, Edwards MJ, et al. Psychogenic palatal tremor may be underrecognized: reappraisal of a large series of cases. Mov Disord. 2012;27:1164–8.
Kern DS, Lang AE. Successful treatment of functional palatal tremor: insights into pathogenesis and management. Mov Disord. 2015;30:875–6. This report highlights the evolving understanding of essential palatal tremor (myoclonus) and its functional etiology.
Nasr A, Brown N. Palatal myoclonus responding to lamotrigine. Seizure. 2002;11:136–7.
Penney SE, Bruce IA, Saeed SR. Botulinum toxin is effective and safe for palatal tremor: a report of five cases and a review of the literature. J Neurol. 2006;253:857–60.
Obeso JA. Therapy of myoclonus. Clin Neurosci. 1995;3:253–7.
Keswani SC, Kossoff EH, Krauss GL, et al. Amelioration of spinal myoclonus with levetiracetam. J Neurol Neurosurg Psychiatry. 2002;73:457–8.
Siniscalchi A, Mancuso F, Russo E, et al. Spinal myoclonus responsive to topiramate. Mov Disord. 2004;19:1380–1.
Chiodo AE, Saval A. Intrathecal baclofen for the treatment of spinal myoclonus: a case series. J Spinal Cord Med. 2012;35:64–7.
Polo KB, Jabbari B. Effectiveness of botulinum toxin type A against painful limb myoclonus of spinal cord origin. Mov Disord. 1994;9:233–5.
Lagueny A, Tison F, Burbaud P, et al. Stimulus-sensitive spinal segmental myoclonus improved with injections of botulinum toxin type A. Mov Disord. 1999;14:182–5.
Vivancos-Matellano F, Arpa-Gutierrez FJ, Perez-Conde MC, et al. The effectiveness of Botulinum toxin type A in two cases of abdominal myoclonias refractory to conventional therapy. Rev Neurol. 2006;42:59–61.
Esposito M, Erro R, Edwards MJ, et al. The pathophysiology of symptomatic propriospinal myoclonus. Mov Disord. 2014;29:1097–9.
Antelmi E, Provini F. Propriospinal myoclonus: the spectrum of clinical and neurophysiological phenotypes. Sleep Med Rev. 2015;22:54–63.
Jang W, Kim JS, Ahn JY, et al. Reversible propriospinal myoclonus due to thoracic disc herniation: long-term follow-up. J Neurol Sci. 2012;313:32–4.
Espay AJ, Ashby P, Hanajima R, et al. Unique form of propriospinal myoclonus as a possible complication of an enteropathogenic toxin. Mov Disord. 2003;18:942–8.
Erro R, Bhatia KP, Edwards MJ, et al. Clinical diagnosis of propriospinal myoclonus is unreliable: an electrophysiologic study. Mov Disord. 2013;28:1868–73. This article highlights that propriospinal myoclonus requires electrophysiological characterization and posists that the etiology in most cases may be functional.
Maltete D, Verdure P, Roze E, et al. TENS for the treatment of propriospinal myoclonus. Mov Disord. 2008;23:2256–7.
Hallett M. Psychogenic movement disorders: neurology and neuropsychiatry. Wolters Kluwer; 2005.
Baizabal-Carvallo JF, Fekete R. Recognizing uncommon presentations of psychogenic (functional) movement disorders. Tremor Other Hyperkinet Mov (N Y). 2015;5:279.
Kompoliti K, Wilson B, Stebbins G, et al. Immediate vs. delayed treatment of psychogenic movement disorders with short term psychodynamic psychotherapy: randomized clinical trial. Parkinsonism Relat Disord. 2014;20:60–3. This article is a well designed randomized cross-over study that demonstrates that patients kept within the medical system and regularly evaluated by physicians with or without psychodynamic therapy can help treat functional movement disorders.
Hinson VK, Weinstein S, Bernard B, et al. Single-blind clinical trial of psychotherapy for treatment of psychogenic movement disorders. Parkinsonism Relat Disord. 2006;12:177–80.
Czarnecki K, Thompson JM, Seime R, et al. Functional movement disorders: successful treatment with a physical therapy rehabilitation protocol. Parkinsonism Relat Disord. 2012;18:247–51.
Dallocchio C, Arbasino C, Klersy C, et al. The effects of physical activity on psychogenic movement disorders. Mov Disord. 2010;25:421–5.
Jordbru AA, Smedstad LM, Klungsoyr O, et al. Psychogenic gait disorder: a randomized controlled trial of physical rehabilitation with one-year follow-up. J Rehabil Med. 2014;46:181–7.
Edwards MJ, Stone J, Nielsen G. Physiotherapists and patients with functional (psychogenic) motor symptoms: a survey of attitudes and interest. J Neurol Neurosurg Psychiatry. 2012;83:655–8.
Zesiewicz TA, Sullivan KL, Arnulf I, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2010;74:924–31.
Kobayashi K, Katayama Y, Otaka T, et al. Thalamic deep brain stimulation for the treatment of action myoclonus caused by perinatal anoxia. Stereotact Funct Neurosurg. 2010;88:259–63.
Yamada K, Sakurama T, Soyama N, et al. Gpi pallidal stimulation for Lance-Adams syndrome. Neurology. 2011;76:1270–2.
Trottenberg T, Meissner W, Kabus C, et al. Neurostimulation of the ventral intermediate thalamic nucleus in inherited myoclonus-dystonia syndrome. Mov Disord. 2001;16:769–71.
Azoulay-Zyss J, Roze E, Welter ML, et al. Bilateral deep brain stimulation of the pallidum for myoclonus-dystonia due to epsilon-sarcoglycan mutations: a pilot study. Arch Neurol. 2011;68:94–8.
Magarinos-Ascone CM, Regidor I, Martinez-Castrillo JC, et al. Pallidal stimulation relieves myoclonus-dystonia syndrome. J Neurol Neurosurg Psychiatry. 2005;76:989–91.
Starr PA. Deep brain stimulation for other tremors, myoclonus, and chorea. Handb Clin Neurol. 2013;116:209–15.
Rughani AI, Lozano AM. Surgical treatment of myoclonus dystonia syndrome. Mov Disord. 2013;28:282–7. This review of published cases compared different targets for functional neurosurgery in myoclonus dystonia syndrome. Both dystonia and myoclonus improved in their analysis. Globus pallidus targets improved dystonia to a greater extent than the thalamic one.
Sidiropoulos C, Mestre T, Hutchison W, et al. Bilateral pallidal stimulation for sargoglycan epsilon negative myoclonus. Parkinsonism Relat Disord. 2014;20:915–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ariel Levy declares no conflict of interest.
Robert Chen received research grant from Medtronic Inc, honorarium from Allergan, and research grant and honorarium from Merz.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Movement Disorders
Rights and permissions
About this article
Cite this article
Levy, A., Chen, R. Myoclonus: Pathophysiology and Treatment Options. Curr Treat Options Neurol 18, 21 (2016). https://doi.org/10.1007/s11940-016-0404-7
Published:
DOI: https://doi.org/10.1007/s11940-016-0404-7