Abstract
Purpose of review
The management of advanced hepatocellular carcinoma (HCC) has drastically changed in the past few years with approval of several first-line and second-line systemic therapies. In this review, we present an overview of the recent progress in the treatment of advanced HCC and discuss future perspectives.
Recent findings
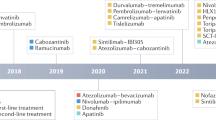
The phase 3 clinical trial IMBRAVE150 has recently shown the combination of an immune checkpoint inhibitor, atezolizumab, with an antiangiogenic agent, bevacizumab, to be superior to sorafenib monotherapy for treatment-naive advanced HCC. Moreover, patients now have multiple options available in second-line therapy including targeted therapies like sorafenib, lenvatinib, regorafenib, cabozantinib, and ramucirumab and immunotherapies like atezolizumab and nivolumab either alone or combined with ipilimumab.
Summary
There has been tremendous recent progress in the management of advanced HCC. Combination therapy with atezolizumab–bevacizumab has recently become the standard first-line therapy for patients with advanced HCC. Additionally, immunotherapy agents are poised to play a significant role in the management of HCC either alone or in combination with molecular targeted therapies.



Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Cancer Today 2020. Global Cancer Observatory: International Agency for Research on Cancer. https://gco.iarc.fr/today/home.
• Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90 This trial led to the approval of sorafenib for the management of advanced HCC.
Yeo W, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–8.
Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, Bianchi L, et al. Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology. 2000;31:54–8.
Ikeda M, Mitsunaga S, Ohno I, Hashimoto Y, Takahashi H, Watanabe K, et al. Systemic chemotherapy for advanced hepatocellular carcinoma: past, present, and future. Diseases. 2015;3:360–81.
• Cheng A-L, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34 This trial led to the approval of sorafenib for the management of advanced HCC.
Cheng A-L, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–75.
Johnson PJ, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–24.
Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33:172–9.
Kudo M, et al. A randomized, double-blind, placebo-controlled phase III study of S-1 in patients with sorafenib-refractory advanced hepatocellular carcinoma (S-CUBE). J Clin Oncol. 2015;33:4018.
• Bruix J, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66 This trial led to the approval of regorafenib as second-line therapy for advanced HCC.
El-Khoueiry AB, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–502.
• Kudo M, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–73 The results of this trial led to the approval of lenvatinib as first-line therapy for advanced HCC.
•• Finn RS, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–905 Results of this early phase trial revealed that immunotherapy can be safe for patients with HCC and can potentially lead to durable responses, leading to its accelerate approval for advanced HCC.
Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52:117–24.
Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624.
Hsu C-H, et al. Randomised efficacy and safety results for atezolizumab (Atezo) bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30:ix187.
Finn RS, et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J Clin Orthod. 2021;39:267.
Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–9.
Jackson R, Psarelli E-E, Berhane S, Khan H, Johnson P. Impact of viral status on survival in patients receiving sorafenib for advanced hepatocellular cancer: a meta-analysis of randomized phase III trials. J Clin Oncol. 2017;35:622–8.
Bruix J, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67:999–1008.
Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, Rimola J, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–24.
Estfan B, Byrne M, Kim R. Sorafenib in advanced hepatocellular carcinoma: hypertension as a potential surrogate marker for efficacy. Am J Clin Oncol. 2013;36:319–24.
Bettinger D, Schultheiβ M, Knüppel E, Thimme R, Blum HE, Spangenberg HC. Diarrhea predicts a positive response to sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2012;56:789–90.
Arao T, et al. FGF3/FGF4 amplification and multiple lung metastases in responders to sorafenib in hepatocellular carcinoma. Hepatology. 2013;57:1407–15.
Personeni N, et al. Molecular determinants of outcome in sorafenib-treated patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2013;139:1179–87.
Marrero JA, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65:1140–7.
McNamara MG, Slagter AE, Nuttall C, Frizziero M, Pihlak R, Lamarca A, et al. Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma-a meta-analysis. Eur J Cancer. 2018;105:1–9.
Abou-Alfa GK, Shi Q, Knox JJ, Kaubisch A, Niedzwiecki D, Posey J, et al. Assessment of treatment with sorafenib plus doxorubicin vs sorafenib alone in patients with advanced hepatocellular carcinoma: phase 3 CALGB 80802 randomized clinical trial. JAMA Oncol. 2019;5:1582. https://doi.org/10.1001/jamaoncol.2019.2792.
Assenat E, et al. Sorafenib alone vs. sorafenib plus GEMOX as 1-line treatment for advanced HCC: the phase II randomised PRODIGE 10 trial. Br J Cancer. 2019;120:896–902.
Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565–75.
Lencioni R, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64:1090–8.
Shimose S, et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: a multicenter cohort study using data mining analysis. Oncol Lett. 2020;20:2257–65.
Cheon J, Chon HJ, Bang Y, Park NH, Shin JW, Kim KM, et al. Real-world efficacy and safety of lenvatinib in Korean patients with advanced hepatocellular carcinoma: a multicenter retrospective analysis. Liver Cancer. 2020;9:613–24.
Amaro CP, Allen MJ, Knox JJ, Tsang ES, Lim HJ, Lee-Ying RM, et al. Efficacy and safety of lenvatinib in the real-world treatment of hepatocellular carcinoma: results from a Canadian multicenter database (HCC CHORD). J Clin Oncol. 2021;39:275.
Lee J, Sung PS, Yang H, Lee SK, Nam HC, Yoo SH, Lee HL, Kim HY, Lee SW, Kwon JH, Jang JW, Kim CW, Nam SW, Bae SH, Choi JY, Yoon SK. A Real-World Comparative Analysis of Lenvatinib and Sorafenib as a Salvage Therapy for Transarterial Treatments in Unresectable HCC. J Clin Med. 2020 Dec 21;9(12):4121. https://doi.org/10.3390/jcm9124121.
National Comprehensive Cancer Network® (NCCN®). NCCN Guidelines for Patients® Liver Cancer. 2020.
Crocenzi TS, et al. Nivolumab (nivo) in sorafenib (sor)-naive and -experienced pts with advanced hepatocellular carcinoma (HCC): CheckMate 040 study. J Clin Oncol. 2017;35:4013.
Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30:v874–5.
Larkin J, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381:1535–46.
Hellmann MD, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–31.
El-Khoueiry AB, et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): long-term results from CheckMate 040. J Clin Orthod. 2021;39:269.
Zhu AX, et al. Pembrolizumab (pembro) in patients with advanced hepatocellular carcinoma (HCC): KEYNOTE-224 update. J Clin Orthod. 2018;36:4020.
Finn RS, et al. KEYNOTE investigators. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs. best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2019;37:4004.
Merle P, et al. Pembrolizumab (pembro) vs placebo (pbo) in patients (pts) with advanced hepatocellular carcinoma (aHCC) previously treated with sorafenib: updated data from the randomized, phase III KEYNOTE-240 study. J Clin Orthod. 2021;39:268.
Merle P, et al. Sequential treatment with sorafenib (SOR) followed by regorafenib (REG) in patients (pts) with unresectable hepatocellular carcinoma (HCC): interim analysis of the observational REFINE study. J Clin Orthod. 2020;38:e16680.
Abou-Alfa GK, Meyer T, Cheng AL, el-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63.
Rimassa L, et al. Outcomes based on age in the phase 3 CELESTIAL trial of cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2018;36:4090.
Kelley RK, et al. Comparative efficacy of cabozantinib and regorafenib for advanced hepatocellular carcinoma. Adv Ther. 2020;37:2678–95.
Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859–70.
• Zhu AX, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–96 The results of this trial led to the approval of ramucirumab for advanced sorafenib experienced HCC in patients with AFP > 400 ng/ml, the first biomarker stratified treatment strategy.
Schutz FAB, Je Y, Richards CJ, Choueiri TK. Meta-analysis of randomized controlled trials for the incidence and risk of treatment-related mortality in patients with cancer treated with vascular endothelial growth factor tyrosine kinase inhibitors. J Clin Oncol. 2012;30:871–7.
Sivendran S, Liu Z, Portas LJ Jr, Yu M, Hahn N, Sonpavde G, et al. Treatment-related mortality with vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy in patients with advanced solid tumors: a meta-analysis. Cancer Treat Rev. 2012;38:919–25.
• Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–8 This paper describes the management of adverse events from systemic therapy for HCC.
Tridente G. Adverse Events and oncotargeted kinase Inhibitors: Academic Press; 2017.
Kim S, Abou-Alfa GK. The role of tyrosine kinase inhibitors in hepatocellular carcinoma. Clin Adv Hematol Oncol. 2014;12:36–41.
Hang XF, Xu WS, Wang JX, Wang L, Xin HG, Zhang RQ, et al. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2011;67:613–23.
Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology. 2010;79:27–38.
Myers G. Immune-related adverse events of immune checkpoint inhibitors: a brief review. Curr Oncol. 2018;25:342–7.
Postow MA, Hellmann MD. Adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:1165.
Brahmer JR, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–68.
Naing A, Hajjar J, Gulley JL, Atkins MB, Ciliberto G, Meric-Bernstam F, et al. Strategies for improving the management of immune-related adverse events. J Immunother Cancer. 2020;8:e001754.
Jeung H-C, Oh SE, Kim JH. Immune-related adverse events: overview and management strategies for the use of immune checkpoint inhibitors. Journal of Rheumatic Diseases. 2019;26:221.
Kelley RK, et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC). J Clin Orthod. 2020;38:4508.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Both T. Tara Ghaziani and Renumathy Dhanasekaran declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Liver
Rights and permissions
About this article
Cite this article
Ghaziani, T.T., Dhanasekaran, R. Recent Progress in Systemic Therapy for Hepatocellular Cancer (HCC). Curr Treat Options Gastro 19, 351–368 (2021). https://doi.org/10.1007/s11938-021-00346-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-021-00346-x