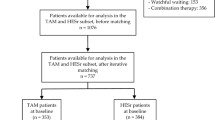
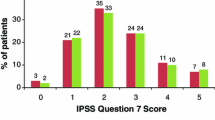
Abstract
Placebo medications and sham surgeries have long been thought to be inert treatments. These groups served as a threshold to which an active treatment should be compared in a randomized trial to determine the true efficacy of the active treatment. However, surprising changes in subjective symptom scores and objective measures of voiding have been demonstrated in numerous placebo medication or sham surgery arms of trials. The exact mechanisms by which these inactive treatments augment patient outcomes are not clearly defined and multiple theories have been proposed to explain the often pronounced response. It appears that urologic outcomes are particularly prone to these effects and the astute physician should keep these responses in mind when interpreting any trial on a new therapy.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McDonald CJ, McCabe GP. How much of the placebo 'effect' is really statistical regression? Stat Med. 1989;8:1301–2.
Fassler M, Meissner K, Kleijnen J, Hrobjartsson A, Linde K. A systematic review found no consistent difference in effect between more and less intensive placebo interventions. J Clin Epidemiol. 2015;68:442–51.
Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–95. A thought provoking and interesting commentary on the phenomenon that is the placebo effect.
Mangera A, Chapple CR, Kopp ZS, Plested M. The placebo effect in overactive bladder syndrome. Nat Rev Urol. 2011;8:495–503.
Welliver C, Kottwitz M, Feustel P, McVary K. Clinically and statistically significant changes seen in sham surgery arms of benign prostatic hyperplasia surgery trials. J Urol. In press.
Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker-Corkery E, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–4.
Barry MJ, Cockett AT, Holtgrewe HL, McConnell JD, Sihelnik SA, Winfield HN. Relationship of symptoms of prostatism to commonly used physiological and anatomical measures of the severity of benign prostatic hyperplasia. J Urol. 1993;150:351–8.
Wang X, Wang X, Li S, et al. Comparative effectiveness of oral drug therapies for lower urinary tract symptoms due to benign prostatic hyperplasia: a systematic review and network meta-analysis. PLoS One. 2014;9:e107593. A recent meta-analysis looking at the “true drug effect” of the commonly used BPH medications along with comparisons between different medications.
van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol. 2000;37:306–13.
Roehrborn CG for the ALFUS study group. Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial. Urology. 2001;58:953–9.
Roehrborn CG, Van Kerrebroeck P, Nordling J. Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies. BJU Int. 2003;92:257–61.
Nordling J. Efficacy and safety of two doses (10 and 15 mg) of alfuzosin or tamsulosin (0.4 mg) once daily for treating symptomatic benign prostatic hyperplasia. BJU Int. 2005;95:1006–12.
Roehrborn CG. Alfuzosin 10 mg once daily prevents overall clinical progression of benign prostatic hyperplasia but not acute urinary retention: results of a 2-year placebo-controlled study. BJU Int. 2006;97:734–41.
Andersen M, Dahlstrand C, Hoye K. Double-blind trial of the efficacy and tolerability of doxazosin in the gastrointestinal therapeutic system, doxazosin standard, and placebo in patients with benign prostatic hyperplasia. Eur Urol. 2000;38:400–9.
McConnell JD, Roehrborn CG, Bautista OM, Andriole Jr GL, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98.
Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003;61:119–26.
Kawabe K, Yoshida M, Homma Y, Silodosin Clinical Study G. Silodosin, a new alpha1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006;98:1019–24.
Chapple CR, Montorsi F, Tammela TL, Wirth M, Koldewijn E, Fernandez Fernandez E, et al. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur Urol. 2011;59:342–52.
Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha(1A)-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol. 2013;189:S122–8.
Lepor H and Tamsulosin Investigator Group. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Urology. 1998;51:892–900.
Lepor H and Tamsulosin Investigator Group. Long-term evaluation of tamsulosin in benign prostatic hyperplasia: placebo-controlled, double-blind extension of phase III trial. Urology. 1998;51:901–6.
Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93–01 Study Group. J Urol. 1998;160:1701–6.
Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319–28.
Kim SC, Park JK, Kim SW, Lee SW, Ahn TY, Kim JJ, et al. Tadalafil admisnistered once daily for the treatment of lower urinary tract symptoms in Korean men with benign prostatic hyperplasia: results from a placebo-controlled pilot study using tamsulosin as an active control. LUTS. 2011;3:86–93.
Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61:917–25.
Yokoyama O, Yoshida M, Kim SC, Wang CJ, Imaoka T, Morisaki Y, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20:193–201.
Lepor H, Williford WO, Barry MJ, Brawer MK, Dixon CM, Gormley G, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996;335:533–9.
Roehrborn CG, Oesterling JE, Auerbach S, Kaplan SA, Lloyd LK, Milam DE, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology. 1996;47:159–68.
Roehrborn CG, Nickel JC, Andriole GL, Gagnier RP, Black L, Wilson TH, et al. Dutasteride improves outcomes of benign prostatic hyperplasia when evaluated for prostate cancer risk reduction: secondary analysis of the REduction by DUtasteride of prostate Cancer Events (REDUCE) trial. Urology. 2011;78:641–6.
Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G, Aria A, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–41.
Na Y, Ye Z, Zhang S, Chinese Dutasteride Phase IIITSG. Efficacy and safety of dutasteride in Chinese adults with symptomatic benign prostatic hyperplasia: a randomized, double-blind, parallel-group, placebo-controlled study with an open-label extension. Clin Drug Investig. 2012;32:29–39.
McConnell JD, Brusketwitz R, Walsh P, Andriole G, Lieber M, Holtgrewe L, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–63.
McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177:1401–7.
Regadas RP, Reges R, Cerqueira JB, Sucupira DG, Josino IR, Nogueira EA, et al. Urodynamic effects of the combination of tamsulosin and daily tadalafil in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: a randomized, placebo-controlled clinical trial. Int Urol Nephrol. 2013;45:39–43.
Porst H, Oelke M, Goldfischer ER, Cox D, Watts S, Dey D, et al. Efficacy and safety of tadalafil 5mg once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: subgroup analysis of pooled data from 4 multinational, randomized, placebo-controlled clinical studies. Urology. 2013;82:667–73.
McVary KT, Monnig W, Camps Jr JL, Young JM, Tseng LJ, van den Ende G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007;177:1071–7.
Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2008;53:1236–44.
Tsukamoto T, Endo Y, Narita M. Efficacy and safety of dutasteride in Japanese men with benign prostatic hyperplasia. Int J Urol. 2009;16:745–50.
Stoner E. Three-year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology. 1994;43:284–92. discussion 92–4.
Byrnes CA, Morton AS, Liss CL, Lippert MC, Gillenwater JY. Efficacy, tolerability, and effect on health-related quality of life of finasteride versus placebo in men with symptomatic benign prostatic hyperplasia: a community based study. CUSP Investigators. Community based study of Proscar. Clin Ther. 1995;17:956–69.
Yu HJ, Chiu TY, Lai MK. Therapeutic effects of finasteride in benign prostatic hyperplasia: a randomized double-blind controlled trial. J Formos Med Assoc. 1995;94:37–41.
Nickel JC, Fradet Y, Boake RC, Pommerville PJ, Perreault JP, Afridi SK, et al. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: results of a 2-year randomized controlled trial (the PROSPECT study). PROscar Safety Plus Efficacy Canadian Two year Study. CMAJ. 1996;155:1251–9.
Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008;180:1228–34.
Dmochowski R, Roehrborn C, Klise S, Xu L, Kaminetsky J, Kraus S. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J Urol. 2010;183:1092–7.
Porst H, Kim ED, Casabe AR, Mirone V, Secrest RJ, Xu L, et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:1105–13.
Madani AH, Afsharimoghaddam A, Roushani A, Farzan A, Asadollahzade A, Shakiba M. Evaluation of tadalafil effect on lower urinary tract symptoms of benign prostatic hyperplasia in patients treated with standard medication. Int Braz J Urol. 2012;38:33–9.
McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–803.
Malaeb BS, Yu X, McBean AM, Elliott SP. National trends in surgical therapy for benign prostatic hyperplasia in the United States (2000–2008). Urology. 2012;79:1111–6.
Nordling J, Abrams P, Ameda K, Andersen JT, Donovan J, Griffiths D, et al. Outcome measures for research in treatment of adult males with symptoms of lower urinary tract dysfunction. Neurourol Urodyn. 1998;17:263–71.
Roehrborn CG, Preminger G, Newhall P, Denstedt J, Razvi H, Perlmutter A, et al. Microwave thermotherapy for benign prostatic hyperplasia with the Dornier Urowave: results of a randomized, double-blind, multicenter, sham-controlled trial. Urology. 1998;51:19–28.
Albala DM, Fulmer BR, Turk TM, Koleski F, Andriole G, Davis BE, et al. Office-based transurethral microwave thermotherapy using the TherMatrx TMx-2000. J Endourol. 2002;16:57–61.
de la Rosette JJ, de Wildt MJ, Alivizatos G, Froeling FM, Debruyne FM. Transurethral microwave thermotherapy (TUMT) in benign prostatic hyperplasia: placebo versus TUMT. Urology. 1994;44:58–63.
Larson TR, Blute ML, Bruskewitz RC, Mayer RD, Ugarte RR, Utz WJ. A high-efficiency microwave thermoablation system for the treatment of benign prostatic hyperplasia: results of a randomized, sham-controlled, prospective, double-blind, multicenter clinical trial. Urology. 1998;51:731–42.
Blute ML, Patterson DE, Segura JW, Tomera KM, Hellerstein DK. Transurethral microwave thermotherapy v sham treatment: double-blind randomized study. J Endourol. 1996;10:565–73.
Roehrborn CG, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. Study. J Urol. 2013;190:2161–7.
Bdesha AS, Bunce CJ, Kelleher JP, Snell ME, Vukusic J, Witherow RO. Transurethral microwave treatment for benign prostatic hypertrophy: a randomised controlled clinical trial. BMJ. 1993;306:1293–6.
Marberger M, Chartier-Kastler E, Egerdie B, Lee KS, Grosse J, Bugarin D, et al. A randomized double-blind placebo-controlled phase 2 dose-ranging study of onabotulinumtoxinA in men with benign prostatic hyperplasia. Eur Urol. 2013;63:496–503.
McVary KT, Roehrborn CG, Chartier-Kastler EM, Bugarin D, Chen R, et al. A multicenter, randomized, double-blind, placebo controlled study of onabotulinumtoxinA 200 U to treat lower urinary tract symptoms in men with benign prostatic hyperplasia. J Urol. 2014;192:150–6.
Maria G, Brisinda G, Civello IM, Bentivoglio AR, Sganga G, Albanese A, et al. Relief by botulinum toxin of voiding dysfunction due to benign prostatic hyperplasia: results of a randomized, placebo-controlled study. Urology. 2003;62:259–64. discussion 264–5.
Elhilali MM, Pommerville P, Yocum RC, Merchant R, Roehrborn CG, Denmeade SR. Prospective, randomized, double-blind, vehicle controlled, multicenter phase IIb clinical trial of the pore forming protein PRX302 for targeted treatment of symptomatic benign prostatic hyperplasia. J Urol. 2013;189:1421–6.
Walach H, Sadaghiani C, Dehm C, Bierman D. The therapeutic effect of clinical trials: understanding placebo response rates in clinical trials—a secondary analysis. BMC Med Res Methodol. 2005;5:26.
Lee S, Malhotra B, Creanga D, Carlsson M, Glue P. A meta-analysis of the placebo response in antimuscarinic drug trials for overactive bladder. BMC Med Res Methodol. 2009;9:55.
Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long-term outcome of medical therapy for BPH. Eur Urol. 2007;51:1522–33.
Nickel JC and Canadian PROSPECT Study Group. Placebo therapy of benign prostatic hyperplasia: a 25-month study. Br J Urol. 1998;81:383–7.
Sech SM, Montoya JD, Bernier PA, Barnboym E, Brown S, Gregory A, et al. The so-called "placebo effect" in benign prostatic hyperplasia treatment trials represents partially a conditional regression to the mean induced by censoring. Urology. 1998;51:242–50.
Compliance with Ethics Guidelines
Conflict of Interest
Igor Sorokin and Adam Schatz each declare no potential conflicts of interest.
Charles Welliver reports other from Sophiris, other from AMS, other from Coloplast, personal fees from American Society of Andrology, other from Antares, other from NexMed, other from Auxilium, and other from Sophiris, and his brother works for Bristol-Myers.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Benign Prostatic Hyperplasia
Rights and permissions
About this article
Cite this article
Sorokin, I., Schatz, A. & Welliver, C. Placebo Medication and Sham Surgery Responses in Benign Prostatic Hyperplasia Treatments: Implications for Clinical Trials. Curr Urol Rep 16, 73 (2015). https://doi.org/10.1007/s11934-015-0544-4
Published:
DOI: https://doi.org/10.1007/s11934-015-0544-4