Abstract
Purpose of Review
Recent studies have investigated the effect of treatments containing adipose-derived mesenchymal stem cells (ADMSCs) on human osteoarthritis. These have mostly used biologic adjuvants which may influence results. Thus, the purpose of this systematic review is to evaluate the current literature on these treatments when used in isolation.
Recent Findings
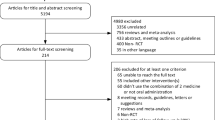
Five studies in this review used cultured ADMSCs, while four studies used stromal vascular fraction and three used micro-fragmented adipose tissue to deliver ADMSCs. No studies reported serious treatment-related adverse effects and all reported improvements in clinical measures for at least one dose. This was not necessarily reflected in imaging evaluations nor were improvements always maintained.
Summary
Current low-level evidence is limited due to variability in study methodology but indicates that treatments containing ADMSCs, when used in isolation, are safe and have the potential to reduce pain and improve function. Randomized controlled trials are now needed.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major Importance
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30.
Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192(1):230–7.
Dequeker J, Luyten FP. The history of osteoarthritis-osteoarthrosis. Ann Rheum Dis. 2008;67(1):5–10.
Mithoefer K, WILLIAMS III RJ, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911–20.
Kaul G, Cucchiarini M, Remberger K, Kohn D, Madry H. Failed cartilage repair for early osteoarthritis defects: a biochemical, histological and immunohistochemical analysis of the repair tissue after treatment with marrow-stimulation techniques. Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2315–24.
Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. JBJS. 1993;75(4):532–53.
Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519–27.
Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthr Cartil. 2011;19(7):779–91.
Marlovits S, Zeller P, Singer P, Resinger C, Vécsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57(1):24–31.
Roberts S, Genever P, McCaskie A, Bari CD. Prospects of stem cell therapy in osteoarthritis. Regen Med. 2011;6(3):351–66.
Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11):e54.
Dominici ML, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26(6):664–75.
Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, et al. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063–77.
•• Hudetz D, Borić I, Rod E, Jeleč Ž, Radić A, Vrdoljak T, et al. The effect of intra-articular injection of autologous microfragmented fat tissue on proteoglycan synthesis in patients with knee osteoarthritis. Genes. 2017;8(10):270. This was one of the first studies to use micro-fragmented fat tissue as a way of delivering ADMSCs.
Ha CW, Park YB. Mesenchymal stem cell injection for osteochondral lesions of the talus: letter to the editor. Am J Sports Med. 2014;42(6):NP34–5.
U.S. Department of Health and Human Services. Regulatory considerations for human cells, tissues, and cellular and tissuebased products: minimal manipulation and homologous use. Available from: https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM585403.pdf. Accessed 5th Aug 2018.
• Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–66. One of the pioneering clinical trials to use ADMSC treatment in isolation. This proof-of-concept clinical trial was followed up at a later time point (see [19••]).
•• Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45(12):2774–83. A recent clinical trial which was one of the few to include a longer term follow-up time point (2 years).
Koh YG, Choi YJ, Kwon OR, Kim YS. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med. 2014;42(7):1628–37.
Kim YS, Choi YJ, Suh DS, Heo DB, Kim YI, Ryu JS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: is fibrin glue effective as a scaffold? Am J Sports Med. 2015;43(1):176–85.
Russo A, Condello V, Madonna V, Guerriero M, Zorzi C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. Journal of experimental orthopaedics. 2017;4(1):33.
Cattaneo G, De Caro A, Napoli F, Chiapale D, Trada P, Camera A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskelet Disord. 2018;19(1):176.
Fodor PB, Paulseth SG. Adipose derived stromal cell (ADSC) injections for pain management of osteoarthritis in the human knee joint. Aesthet Surg J. 2015;36(2):229–36.
Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–56.
•• Spasovski D, Spasovski V, Baščarević Z, Stojiljković M, Vreća M, Anđelković M, et al. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med. 2018;20(1):e3002. A comprehensive study that assessed a range of outcome measures at several follow-ups, after injection with ADMSCs.
•• Yokota N, Yamakawa M, Shirata T, Kimura T, Kaneshima H. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther. 2017;6:108–12. A recent study that highlighted the potential of treatments that deliver ADMSCs via SVF for osteoarthritis.
• Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, et al. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13(3):295–307. A pilot study which was one of the first in the field to explore the effects of multiple injections on clinical outcomes after ADMSC treatment.
Kim YS, Choi YJ, Koh YG. Mesenchymal stem cell implantation in knee osteoarthritis: an assessment of the factors influencing clinical outcomes. Am J Sports Med. 2015;43(9):2293–301.
Gray ML, Burstein D, Kim YJ, Maroudas A. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, imaging technique, and clinical applications. J Orthop Res. 2008;26(3):281–91.
Williams A, Gillis A, McKenzie C, Po B, Sharma L, Micheli L, et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. Am J Roentgenol. 2004;182(1):167–72.
Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng A. 2012;18(11–12):1161–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Complementary and Alternative Medicine.
Rights and permissions
About this article
Cite this article
Ranmuthu, C.D.S., Ranmuthu, C.K.I. & Khan, W.S. Evaluating the Current Literature on Treatments Containing Adipose-Derived Stem Cells for Osteoarthritis: a Progress Update. Curr Rheumatol Rep 20, 67 (2018). https://doi.org/10.1007/s11926-018-0776-7
Published:
DOI: https://doi.org/10.1007/s11926-018-0776-7