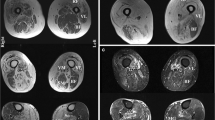
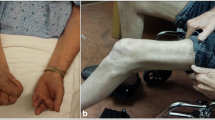
Abstract
Purpose of Review
While sporadic inclusion body myositis (sIBM) is the most common acquired muscle disease after age 50, the pathogenesis of this disease is still poorly understood. In this review, we discuss our current state of knowledge in sIBM and provide an update on our current understanding of its pathophysiology and management.
Recent Findings
Lines of evidence in support of an inflammatory pathogenesis include inflammatory infiltrates in the target organ, NFκB activation, cytokine response, MHC I upregulation, and cN1A antibody. Refractoriness to immunotherapies has led to suggestion of a degenerative pathophysiology. Evidence for impaired protein homeostasis with misfolding burden is coupled with findings of endoplasmic reticulum stress, proteasome dysfunction, and mitochondrial lesion. Recent treatment trials have focused more on correcting the degenerative process or muscle growth rather than controlling the inflammation.
Summary
There has been growing evidence toward degeneration as the primary process in sIBM. This is consistent with the refractory nature of this disease. Improving our understanding of this disease pathogenesis will propel efforts to find an effective therapy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Amato AA, Barohn RJ. Inclusion body myositis: old and new concepts. J Neurol Neurosurg Psychiatry. 2009;80(11):1186–93.
Felice KJ, North WA. Inclusion body myositis in Connecticut: observation in 35 patients during an 8-year period. Medicine (Baltimore). 2001;80:320–7.
Wilson FC, Ytterberg SR, St Sauver JL, Reed AM. Epidemiology of sporadic inclusion body myositis and polymyositis and Olmsted County, Minnesota. J Rheumatol. 2008;35:445–7.
Dimachkie MM, Barohn RJ. Inclusion body myositis. Curr Neurol Neurosci Rep. 2013;13:321.
Cox FM, Titulaer MJ, Sont JK, Wintzen AR, Verschuuren JJ, Badrising UA. A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain. 2011;134(Pt 11):3167–75.
Lilleker JB, Rietveld A, Pye SR, Mariampillai K, Benveniste O, Peeters MT, et al. Cytosolic 5-nucleotidase 1A autoantibody profile and clinical characteristics in inclusion body myositis. Ann Rheum Dis. 2017;76(5):862–8.
Needham M, James I, Corbett A, Day T, Christiansen F, Philips B, et al. Sporadic inclusion body myositis: phenotypic variability and influence of HLA-DR3 in a cohort of 57 Australian cases. J Neurol Neurosurg Psychiatry. 2008;79:1056–60.
Amato AA, Gronseth GS, Jackson CE, Wolfe GI, Katz JS, Bryan WW, et al. Inclusion body myositis: clinical and pathological boundaries. Ann Neurol. 1996;40:581–6.
Rose MR; ENMC IBM Working Group. 188th ENMC international workshop: inclusion body myositis, 2–4 December 2011, Naarden, The Netherlands. Neuromuscul Disord. 2013;23:1044–55.
Weir S, Dimachkie MM, Mozaffar et al. Sensitivity of IBM criteria in multicenter cohort. Journal of Clinical Neuromuscular Disease (Suppl) 2018; 19(3):S27.
Lloyd TE, Mammen AL, Amato AA, Weiss MD, Needham M, Greenberg SA. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. 2014;83(5):426–33.
Ma H, McEvoy KM, Milone M. Sporadic inclusion body myositis presenting with severe camptocormia. J Clin Neurosci. 2013;20(11):1628–9.
Finesterer J, Stollberger C, Kovacs GG. Asymptomatic hyper-creatine-kinase-emia as sole manifestation of inclusion body myositis. Neurol Int. 2013;5(2):34–6.
Dimachkie MM, Barohn RJ. Inclusion body myositis. Neurol Clin. 2014;32(3):629–46.
Rodriguez Cruz PM, Needham M, Hollingsworth P, Mastagia FL, Hillman DR. Sleep disordered breathing and subclinical impairment of respiratory function are common in sporadic inclusion body myositis. Neuromuscul Disord. 2014;24(12):1036–41.
Teixeira A, Cherin P, Demoule A, Levy-Soussan M, Straus C, Verin E, et al. Diaphragmatic dysfunction in patients with idiopathic inflammatory myopathies. Neuromuscul Disord. 2005;15(1):32–9.
Pluk H, van Hoeve BJ, van Dooren SH, Stammen-Volgelzangs J, van der Heijden A, Schelhaas HJ, et al. Autoantibodies to cytosolic 5-nucleotidase 1A in inclusion body myositis. Ann Neurol. 2013;73(3):397–407.
Larman HB, Salajegheh M, Nazareno R, Lam T, Sauld J, Steen H, et al. Cytosolic 5-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol. 2013;73(3):408–18.
• Herbert MK, Stammen-Vogelzangs J, Verbeek MM, et al. Disease specificity of autoantibodies to cytosolic 5-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Ann Rheum Dis. 2016;75(4):696–701. This study evaluated the specificity of cN1A antibody to IBM as compared to autoimmune disorders.
•• Lloyd TE, Christopher-Stine L, Pinal-Fernandez I, et al. Cytosolic 5-nucleotidase 1A as a target of circulating autoantibodies in autoimmune diseases. Arthritis Care Res (Hoboken). 2016;68(1):66–71. This study reveals the presence of cN1A antibody in other inflammatory myopathies.
Catalan-Garcia M, Garrabou G, Moren C, Guitart-Mampel M, Gonzalez-Casacuberta I, Hernando A, et al. BACE-1, PS-1and sAPPβ levels are increased in plasma from sporadic inclusion body myositis patients: surrogate biomarkers among inflammatory myopathies. Mol Med. 2015; https://doi.org/10.2119/molmed.2015.00168.
Cox FM, Reijnierse M, van Rijswijk CS, Wintzen AR, Verschuuren JJ, Badrising UA. Magnetic resonance imaging of skeletal muscles in sporadic inclusion body myositis. Rheumatology (Oxford). 2011;50(6):1153–61.
Tasca G, Monforte M, De Fino C, Kley RA, Ricci E, Mirabella M. Magnetic resonance imaging pattern recognition in sporadic inclusion-body myositis. Muscle Nerve. 2015;52(6):956–62.
Hiniker A, Daniels BH, Lee HS, Margeta M. Comparative utility of LC3, p62 and TDP-43 immunohistochemistry in differentiation of inclusion body myositis from polymyositis and related inflammatory myopathies. Acta Neuropathol Commun. 2013;1:29.
Griggs RC, Askanas V, DiMauro S, Engel A, Karpati G, Mendell JR, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995;38:705–13.
Brady S, Squier W, Hilton-Jones D. Clinical assessment determines the diagnosis of inclusion body myositis independently. J Neurol Neurosurg Psychiatry. 2013;84(11):1240–6.
Jackson CE, Barohn RJ, Gronseth G, Pandya S, Herbelin L, Muscle Study Group. Inclusion body myositis functional rating scale: a reliable and valid measure of disease severity. Muscle Nerve. 2008;37(4):473–6.
Lloyd TE, Pinal-Fernandez I, Michelle EH, Christopher-Stine L, Pak K, Sacktor N, et al. Overlapping features of polymyositis and inclusion body myositis in HIV-infected patients. Neurology. 2017;88(15):1454–60.
Rothwell S, Lilleker JB, Lamb JA. Genetics in inclusion body myositis. Curr Opin Rheumatol. 2017;29(6):639–44.
Rothwell S, Cooper RG, Lundberg IE, Gregersen PK, Hanna MG, Machado PM, et al. Immune-Array analysis in sporadic inclusion body myositis reveals HLA-DRB1 amino acid heterogeneity across the myositis spectrum. Arthritis Rheumatol. 2017;69(5):1090–9.
Greenberg SA, Pinkus JL, Amato AA, Kristensen T, Dorfman DM. Association of inclusion body myositis with T cell large granular lymphocytic leukemia. Brain. 2016;139:1348–60.
Barohn RJ, Amato AA, Sahenk Z, Kissel JT, Mendell JR. Inclusion body myositis: explanation for poor response to immunosuppressive therapy. Neurology. 1995;45:1302–4.
Mendell JR, Sahenk Z, Gales T, Paul L. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch Neurol. 1991;48:1229–34.
Askanas V, Engel WK. Inclusion-body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition. Neurology. 2006;66(2 Suppl 1):S39–48.
Nogalska A, D’Agostino C, Engel WK, Klein WL, Askanas V. Novel demonstration of amyloid-β oligomers in sporadic inclusion-body myositis muscle fibers. Acta Neuropathol. 2010;120(5):661–6.
Gang Q, Bettencourt C, Machado PM, Fox Z, Brady S, Healy E, Parton M, Holton JL, Hilton-Jones D, Shieh PB, Zanoteli E, de Paepe B, de Bleecker J, Shaibani A, Ripolone M, Violano R, Moggio M, Barohn RJ, Dimachkie MM, Mora M, Mantegazza R, Zanotti S, Hanna MG, Houlden H, Muscle Study Group and the International IBM Genetics Consortium The effect of an intronic polymorphism in TOMM40 and APOE genotypes in sporadic inclusion body myositis. Neurobiol Aging 2015;36(4):1766.e1–1761766.e3.
Salajegheh M, Pinkus JL, Taylor JP, Amato AA, Nazareno R, Baloh RH, et al. Sarcoplasmic redistribution of nuclear TDP-43 in inclusion body myositis. Muscle Nerve. 2009;40(1):19–31.
Ikenaga C, Kubota A, Kadoya M, Taira K, Uchio N, Hida A, et al. Clinicopathologic features of myositis patients with CD8-MHC-1 complex pathology. Neurology. 2017;89(10):1060–8.
• Gang Q, Bettencourt C, Machado PM, et al. Rare variants in SQSTM1 and VCP genes and risk of sporadic inclusion body myositis. Neurobiol Aging. 2016;47:218.e1–208.e9. This study suggests genetic susceptibility affecting neurodegenerative pathways in sIBM.
•• Ahmed M, Machado PM, Miller A, Spicer C, Herbelin L, He J, Noel J et al. Targeting protein homeostasis in sporadic inclusion body myositis. Sci Trans Med 2016;8(331):331ra41. This article describes the effect of targeting heat shock response in IBM and the available data on arimoclomol.
Benveniste O, Guiguet M, Freebody J, Dubourg O, Squier W, Maisonobe T, et al. Long-term observational study of sporadic inclusion body myositis. Brain. 2011;134(Pt 11):3176–84.
Badrising UA, Maat-Schieman ML, Ferrari MD, Zwinderman AH, Wessels JA, Breedveld FC, et al. Comparison of weakness progression in inclusion body myositis during treatment with methotrexate or placebo. Ann Neurol. 2002;51(3):369–72.
Amato AA, Barohn RJ, Jackson CE, Pappert EJ, Sahenk Z, Kissel JT. Inclusion body myositis: treatment with intravenous immunoglobulin. Neurology. 1994;44(8):1516–8.
Muscle Study Group. Randomized pilot trial of betaINF1a (Avonex) in patients with inclusion body myositis. Neurology. 2001;57:1566–70.
Barohn RJ, Herbelin L, Kissel JT, King W, McVey AL, Saperstein DS, et al. Pilot trial of etanercept in the treatment of inclusion-body myositis. Neurology. 2006;66(2 Suppl 1):S123–4.
Saperstein DS, Levine T, Hank N, et al. Pilot trial of lithium treatment in inclusion body myositis. Neurology. 2011;76(Suppl 4):A106.
Kosmidis ML, Alexopoulos H, Tzioufas AG, Dalakas MC. The effect of anakinra, an IL1 receptor antagonist, in patients with sporadic inclusion body myositis (sSIBM): a small pilot study. J Neurol Sci. 2013;334(1–2):123–5.
Amato AA, Sivakumar K, Goyal N, David WS, Salajegheh M, Praestgaard J, et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology. 2014;83(24):2239–46.
Amato AA, Badrising U, Benveniste O et al. A randomized, double-blind, placebo-controlled study of bimagrumab in patients with sporadic inclusion body myositis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10).
• Mendell JR, Sahenk Z, Al-Zaidy S, Rodino-Klapac LR, Lowes LP, Alfano LN, et al. Follistatin gene therapy for sporadic inclusion body myositis improves functional outcomes. Mol Ther. 2017;25(4):870–9. This study evaluates the possible benefit of targeting myostatin through gene therapy in sIBM.
Arnardottir S, Alexanderson H, Lundberg IE, Borg K. Sporadic inclusion body myositis: pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reaction. J Rehabil Med. 2003;35(1):31–5.
Johnson LG, Collier KE, Edwards DJ, Philippe DL, Eastwood PR, Walters SE, et al. Improvement in aerobic capacity after an exercise program in sporadic inclusion body myositis. J Clin Neuromuscul Dis. 2009;10(4):178–84.
Alexanderson H, Lundberg IE. Exercise as a therapeutic modality in patients with idiopathic inflammatory myopathies. Curr Opon Rheumatol. 2012;24(2):201–7.
Murata KY, Kouda K, Tajima F, Kondo T. A dysphagia study in patients with sporadic inclusion body myositis (s-IBM). Neurol Sci. 2012;33(4):765–70.
Langdon PC, Mulcahy K, Shepherd KL, Low VH, Mastaglia FL. Pharyngeal dysphagia in inflammatory muscle diseases resulting from impaired suprahoid musculature. Dysphagia. 2012;27(3):408–17.
• Schrey A, Airas L, Jokela M, Pulkkinen J. Botulinum toxin alleviates dysphagia of patients with inclusion body myositis. J Neurol Sci. 2017;380:142–7. This study examines the efficacy of botulinum toxin injection in treating dysphagia and reducing aspiration risk in sIBM.
Cortese A, Machado P, Morrow J, Dewar L, Hiscock A, Miller A, et al. Longitudinal observational study of sporadic inclusion body myositis: implications for clinical trials. Neuromuscul Disord. 2013;23(5):404–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Barohn reports personal fees from NuFactor, Plan 365 Inc., Novartis Pharmaceuticals, Option Care, and PlatformQ Health Education and grants from Eli Lilly and Company, PTC Therapeutics, Cytokinetics, Inc., Neuraltus Pharmaceuticals, Inc., Alexion Pharmaceuticals, Inc., ALSA, MDA-Myotonic Dystrophy Foundation, The Marigold Foundation, Sarepta Therapeutics, Ionis Pharmaceuticals, TEVA Pharmaceuticals, Biomarin, Sanofi/Genzyme, NIH, FDA/OOPD, and outside the submitted work.
Dr. Dimachkie is a consultant or on the speaker’s bureau for Alnylam, Audentes, Biomarin, Catalyst, CSL-Behring, Genzyme, Mallinckrodt, Novartis, NuFactor, Octapharma, Sanofi, Shire and Terumo. Dr. Dimachkie received grants from Alexion, Alnylam, Amicus, Biomarin, Bristol-Myers Squibb, Catalyst, CSL-Behring, FDA/OPD, GlaxoSmithKline, Genentech, Grifols, MDA, NIH, Novartis, Genzyme, Octapharma, UCB Biopharma, Viromed and TMA.
Drs. Jabari and Vedanarayanan have nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Inflammatory Muscle Disease
Rights and permissions
About this article
Cite this article
Jabari, D., Vedanarayanan, V.V., Barohn, R.J. et al. Update on Inclusion Body Myositis. Curr Rheumatol Rep 20, 52 (2018). https://doi.org/10.1007/s11926-018-0755-z
Published:
DOI: https://doi.org/10.1007/s11926-018-0755-z