Abstract
Purpose of Review
Bone turnover is a regulated process. Osteoglycin is suggested to have an important impact on bone function but may also affect cardiovascular and metabolic functions. This review investigates the action of osteoglycin in bone as well as its potential endocrine effects.
Recent Findings
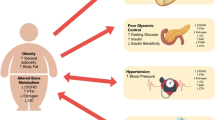
Osteoglycin is expressed by several tissues including bone and muscle. Some studies suggest that osteoglycin increases osteoblast differentiation whereas others suggest that osteoglycin decreases osteoblast differentiation. Thus, findings on the influence of osteoglycin in bone are conflicting. A recent study found increased bone mass in osteoglycin deficient mice. Another study reported that osteoglycin is a marker of low bone mineral density and vertebral fractures in women with type 2 diabetes. Furthermore, clinical studies link osteoglycin to insulin resistance and cardiovascular disease.
Summary
Osteoglycin may be a novel marker of a muscle, pancreatic, and bone axis. However, current evidence is limited and further research investigating osteoglycin in both a pre-clinical and a clinical setting is needed.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Westberg-Rasmussen S, Starup-Linde J, Hermansen K, Holst JJ, Hartmann B, Vestergaard P, et al. Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects. Bone. 2017 Jan 23;97:261–6.
Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274(27):18843–6.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Boskey AL. Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep. 2013;2:447.
Delmas PD. What do we know about biochemical bone markers? Baillieres Clin Obstet Gynaecol. 1991;5(4):817–30.
Garnero P. Bone markers in osteoporosis. Curr Osteoporos Rep. 2009 Sep;7(3):84–90.
Szulc P, Bauer DC, Eastell R editors. Primer on the metabolic bone diseases and disorders of mineral metabolism chapter 35, biochemical markers of bone turnover in osteoporosis (pages 297–306). Eighth Edition, Editor(s): Clifford J. Rosen ed. Print ISBN: 9781118453889, Online ISBN: 9781118453926, DOI: https://doi.org/10.1002/9781118453926; 19 JUL 2013.
Imai Y, Youn MY, Inoue K, Takada I, Kouzmenko A, Kato S. Nuclear receptors in bone physiology and diseases. Physiol Rev. 2013;93(2):481–523.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42.
Pierce AM, Lindskog S, Hammarstrom L. Osteoclasts: structure and function. Electron Microsc Rev. 1991;4(1):1–45.
Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011 Feb;343(2):289–302.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011 Feb;26(2):229–38.
Hall JE, Guyton AC. Guyton and Hall textbook of medical physiology. 12nd ed. Philadelphia: Saunders/Elsevier; 2011.
Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5.
Galluzzi F, Stagi S, Salti R, Toni S, Piscitelli E, Simonini G, et al. Osteoprotegerin serum levels in children with type 1 diabetes: a potential modulating role in bone status. Eur J Endocrinol. 2005;153(6):879–85.
Manolagas SC, Almeida M. Gone with the Wnts: beta-catenin, T-cell factor, forkhead box O, and oxidative stress in age-dependent diseases of bone, lipid, and glucose metabolism. Mol Endocrinol. 2007;21(11):2605–14.
Madisen L, Neubauer M, Plowman G, Rosen D, Segarini P, Dasch J, et al. Molecular cloning of a novel bone-forming compound: osteoinductive factor. DNA Cell Biol. 1990;9(5):303–9.
Funderburgh JL, Corpuz LM, Roth MR, Funderburgh ML, Tasheva ES, Conrad GW. Mimecan, the 25-kDa corneal keratan sulfate proteoglycan, is a product of the gene producing osteoglycin. J Biol Chem. 1997;272(44):28089–95.
Yang CH, Culshaw GJ, Liu MM, Lu CC, French AT, Clements DN, et al. Canine tissue-specific expression of multiple small leucine rich proteoglycans. Vet J. 2012;193(2):374–80.
Zhu F, Friedman MS, Luo W, Woolf P, Hankenson KD. The transcription factor osterix (SP7) regulates BMP6-induced human osteoblast differentiation. J Cell Physiol. 2012;227(6):2677–85.
Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol. 2010;658:43–9.
Zhou X, Zhang Z, Feng JQ, Dusevich VM, Sinha K, Zhang H, et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc Natl Acad Sci U S A. 2010;107(29):12919–24.
Tanaka K, Matsumoto E, Higashimaki Y, Katagiri T, Sugimoto T, Seino S, et al. Role of osteoglycin in the linkage between muscle and bone. J Biol Chem. 2012;287(15):11616–28.
Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus - a systematic review. Bone. 2015.
• Chen X, Chen J, Xu D, Zhao S, Song H, Peng Y. Effects of osteoglycin (OGN) on treating senile osteoporosis by regulating MSCs. BMC Musculoskelet Disord. 2017;18(1):423-017–1779-7. Osteoglycin over-expression increases bone formation markers in bone marrow mesenchymal stem cells.
Tanaka K, Kanazawa I, Yamaguchi T, Yano S, Kaji H, Sugimoto T. Active vitamin D possesses beneficial effects on the interaction between muscle and bone. Biochem Biophys Res Commun. 2014;450(1):482–7.
Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23(4):1225–34.
Patel MJ, Chang KH, Sykes MC, Talish R, Rubin C, Jo H. Low magnitude and high frequency mechanical loading prevents decreased bone formation responses of 2T3 preosteoblasts. J Cell Biochem. 2009;106(2):306–16.
Ge G, Seo NS, Liang X, Hopkins DR, Hook M, Greenspan DS. Bone morphogenetic protein-1/tolloid-related metalloproteinases process osteoglycin and enhance its ability to regulate collagen fibrillogenesis. J Biol Chem. 2004;279(40):41626–33.
Tasheva ES, Koester A, Paulsen AQ, Garrett AS, Boyle DL, Davidson HJ, et al. Mimecan/osteoglycin-deficient mice have collagen fibril abnormalities. Mol Vis. 2002;8:407–15.
•• Lee NJ, Ali N, Zhang L, Qi Y, Clarke I, Enriquez RF, et al. Osteoglycin, a novel coordinator of bone and glucose homeostasis. Mol Metab. 2018;13:30–44. In this study osteoglycin is shown to regulate insulin secretion and insulin resistance. Furthermore osteoglycin deficient mice presented with increased bone mass.
•• Tanaka KI, Kanazawa I, Kaji H, Sugimoto T. Association of osteoglycin and FAM5C with bone turnover markers, bone mineral density, and vertebral fractures in postmenopausal women with type 2 diabetes mellitus. Bone. 2017;95:5–10. Circulating osteoglycin in humans is associated with prevalent vertebral fractures and low bone mineral density.
Bendix EF, Johansen E, Ringgaard T, Wolder M, Starup-Linde J. Diabetes and abdominal aortic calcification-a systematic review. Curr Osteoporos Rep. 2018;16(1):42–57.
Deckx S, Heymans S, Papageorgiou AP. The diverse functions of osteoglycin: a deceitful dwarf, or a master regulator of disease? FASEB J. 2016;30(8):2651–61.
Shanahan CM, Cary NR, Osbourn JK, Weissberg PL. Identification of osteoglycin as a component of the vascular matrix. Differential expression by vascular smooth muscle cells during neointima formation and in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1997;17(11):2437–47.
Petretto E, Sarwar R, Grieve I, Lu H, Kumaran MK, Muckett PJ, et al. Integrated genomic approaches implicate osteoglycin (Ogn) in the regulation of left ventricular mass. Nat Genet. 2008;40(5):546–52.
Van Aelst LN, Voss S, Carai P, Van Leeuwen R, Vanhoutte D, Sanders-van Wijk S, et al. Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ Res. 2015;116(3):425–36.
Jazbutyte V, Fiedler J, Kneitz S, Galuppo P, Just A, Holzmann A, et al. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age (Dordr). 2013;35(3):747–62.
Yang Y, Wu QH, Li Y, Gao PJ. Association of SLRPs with carotid artery atherosclerosis in essential hypertensive patients. J Hum Hypertens. 2018;32(8–9):564–71.
• Deckx S, Heggermont W, Carai P, Rienks M, Dresselaers T, Himmelreich U, et al. Osteoglycin prevents the development of age-related diastolic dysfunction during pressure overload by reducing cardiac fibrosis and inflammation. Matrix Biol. 2018;66:110–24. This study suggests that osteoglycin protects from diastolic dysfunction due to cardiac fibrosis, which developed in osteoglycin deficient mice.
Cheng JM, Akkerhuis KM, Meilhac O, Oemrawsingh RM, Garcia-Garcia HM, van Geuns RJ, et al. Circulating osteoglycin and NGAL/MMP9 complex concentrations predict 1-year major adverse cardiovascular events after coronary angiography. Arterioscler Thromb Vasc Biol. 2014 May;34(5):1078–84.
• Baek SH, Cha RH, Kang SW, Park CW, Cha DR, Kim SG, et al. Higher serum levels of osteoglycin are associated with all-cause mortality and cardiovascular and cerebrovascular events in patients with advanced chronic kidney disease. Tohoku J Exp Med. 2017;242(4):281–90. In this study osteoglycin is associated with cardiovascular events and mortality and may thus be a marker of cardiovascular disease.
• Tamura Y, Fujito H, Kawao N, Kaji H. Vitamin D deficiency aggravates diabetes-induced muscle wasting in female mice. Diabetol Int. 2016;8(1):52–8 Vitamin D deficiency decreased the expression of osteoglycin in mice.
Insenser M, Montes-Nieto R, Vilarrasa N, Lecube A, Simo R, Vendrell J, et al. A nontargeted proteomic approach to the study of visceral and subcutaneous adipose tissue in human obesity. Mol Cell Endocrinol. 2012;363(1–2):10–9.
Madsen LR, Baggesen LM, Richelsen B, Thomsen RW. Effect of Roux-en-Y gastric bypass surgery on diabetes remission and complications in individuals with type 2 diabetes: a Danish population-based matched cohort study. Diabetologia. 2019;6:611–20.
Dasch JR, Pace DR, Avis PD, Bentz H, Chu S. Characterization of monoclonal antibodies recognizing bovine bone osteoglycin. Connect Tissue Res. 1993;30(1):11–21.
Hygum K, Starup-Linde J, Harslof T, Vestergaard P, Langdahl BL. Mechanisms in endocrinology: diabetes mellitus, a state of low bone turnover - a systematic review and meta-analysis. Eur J Endocrinol 2017;176(3):R137-R157.
Starup-Linde J, Frost M, Vestergaard P. Abrahamsen B. Calcif Tissue Int: Epidemiology of fractures in diabetes; 2016.
Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes--a meta-analysis. Osteoporos Int. 2007;18(4):427–44.
Fuglsang-Nielsen R, Starup-Linde J, Gregersen S, Vestergaard P. The effect of meals on bone turnover - a systematic review with focus on diabetic bone disease. Expert Rev Endocrinol Metab. 2018;13(5):233–49.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jakob Starup-Linde reports personal fees from Gilead Sciences Denmark and Eli Lilly Denmark.
Rikke Viggers and Aase Handberg declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Bone and Diabetes
Rights and permissions
About this article
Cite this article
Starup-Linde, J., Viggers, R. & Handberg, A. Osteoglycin and Bone—a Systematic Review. Curr Osteoporos Rep 17, 250–255 (2019). https://doi.org/10.1007/s11914-019-00523-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-019-00523-z