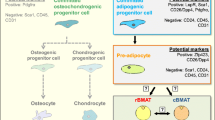
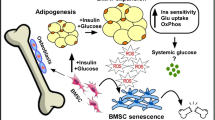
Abstract
Purpose of review
The goal of this review is to gain a better understanding of marrow adipocyte development, its regulation of energy, and its characterization responsible for bone homeostasis.
Recent findings
Despite major advances in uncovering the complex association of bone-fat in the marrow, the underlying basic biological process of adipose tissue development, as well as its interaction with bone homeostasis in pathophysiological conditions, is still not well understood.
Summary
This review identifies many pro- and anti-osteogenic factors secreted by adipocytes to play a role in the manipulating the fate of mesenchymal stem cells as well as the osteoblastic activity during bone remodeling. It also addresses the function of adipose tissue capable of negative regulation of the hematopoietic microenvironment to influence the bone quantity and the nature of bone homeostasis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Ghali O, Al RN, Hardouin P, Chauveau C. Increased bone marrow adiposity in a context of energy deficit: the tip of the iceberg? Front Endocrinol (Lausanne). 2016;7:125. https://doi.org/10.3389/fendo.2016.00125.
Hardouin P, Rharass T, Lucas S. Bone marrow adipose tissue: to be or not to be a typical adipose tissue? Front Endocrinol. 2016;7:85. https://doi.org/10.3389/fendo.2016.00085.
•• Lecka-Czernik B, Stechschulte LA, Czernik PJ, Sherman SB, Huang S, Krings A. Marrow adipose tissue: skeletal location, sexual dimorphism, and response to sex steroid deficiency. Front Endocrinol. 2017;8:188. https://doi.org/10.3389/fendo.2017.00188. The paper reveals the close relationship exists between bone marrow beige adipocytes and bone mass in limbs and sex steroid deficiency also impacts this positive correlation.
Baum T, Cordes C, Dieckmeyer M, Ruschke S, Franz D, Hauner H, et al. MR-based assessment of body fat distribution and characteristics. Eur J Radiol. 2016;85(8):1512–8. https://doi.org/10.1016/j.ejrad.2016.02.013.
Bartelt A, Beil FT, Schinke T, Roeser K, Ruether W, Heeren J, et al. Apolipoprotein E-dependent inverse regulation of vertebral bone and adipose tissue mass in C57Bl/6 mice: modulation by diet-induced obesity. Bone. 2010;47(4):736–45. https://doi.org/10.1016/j.bone.2010.07.002.
Scheller EL, Cawthorn WP, Burr AA, Horowitz MC, MacDougald OA. Marrow adipose tissue: trimming the fat. Trends Endocrinol Metab. 2016;27(6):392–403. https://doi.org/10.1016/j.tem.2016.03.016.
Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–52. https://doi.org/10.1016/j.bone.2011.06.016.
Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846–55. https://doi.org/10.1172/JCI19900.
Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–56.
•• Wu M, Wang Y, Shao J, Wang J, Chen W, Li Y. Cbfβ governs osteoblast−adipocyte lineage commitment through enhancing β-catenin signaling and suppressing adipogenesis gene expression. Proc Natl Acad Sci. 2017;114(38):10119–24. https://doi.org/10.1073/pnas.1619294114. This research suggests that the osteoblast lineage commitment is regulated by core-binding factor subunit beta (Cbfβ) through enhancing β-catenin signaling in mesenchymal stem cells.
Post S, Abdallah BM, Bentzon JF, Kassem M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008;43(1):32–9. https://doi.org/10.1016/j.bone.2008.03.011.
Crossno JJ, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116(12):3220–8. https://doi.org/10.1172/JCI28510.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7. https://doi.org/10.1038/nature07182.
Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13(12):1496–503. https://doi.org/10.1038/nm1672.
Iwamoto I, Fujino T, Douchi T. The leptin receptor in human osteoblasts and the direct effect of leptin on bone metabolism. Gynecol Endocrinol. 2004;19(2):97–104.
Kapur S, Amoui M, Kesavan C, Wang X, Mohan S, Baylink DJ, et al. Leptin receptor (Lepr) is a negative modulator of bone mechanosensitivity and genetic variations in Lepr may contribute to the differential osteogenic response to mechanical stimulation in the C57BL/6J and C3H/HeJ pair of mouse strains. J Biol Chem. 2010;285(48):37607–18. https://doi.org/10.1074/jbc.M110.169714.
Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175(2):405–15.
Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, et al. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28(1):22–34. https://doi.org/10.1002/jbmr.1734.
Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26(6):618–23. https://doi.org/10.1007/s00774-008-0861-5.
Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, et al. Adipokines and the risk of fracture in older adults. J Bone Miner Res. 2011;26(7):1568–76. https://doi.org/10.1002/jbmr.361.
Motyl KJ, Rosen CJ. Understanding leptin-dependent regulation of skeletal homeostasis. Biochimie. 2012;94(10):2089–96. https://doi.org/10.1016/j.biochi.2012.04.015.
Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50(2):534–9. https://doi.org/10.1016/j.bone.2011.06.032.
Philbrick KA, Wong CP, Branscum AJ, Turner RT, Iwaniec UT. Leptin stimulates bone formation in ob/ob mice at doses having minimal impact on energy metabolism. J Endocrinol. 2017;232(3):461–74. https://doi.org/10.1530/JOE-16-0484.
Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35(4):842–9. https://doi.org/10.1016/j.bone.2004.06.008.
Pacheco-Pantoja EL, Waring VJ, Wilson PJ, Fraser WD, Gallagher JA. Adiponectin receptors are present in RANK-L-induced multinucleated osteoclast-like cells. J Recept Signal Transduct Res. 2013;33(5):291–7. https://doi.org/10.3109/10799893.2013.828070.
Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, et al. The effects of adiponectin and leptin on changes in bone mineral density. Osteoporos Int. 2012;23(6):1699–710. https://doi.org/10.1007/s00198-011-1768-x.
Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20(2):368–75. https://doi.org/10.1016/j.cmet.2014.06.003.
Pararasa C, Bailey CJ, Griffiths HR. Ageing, adipose tissue, fatty acids and inflammation. Biogerontology. 2015;16(2):235–48. https://doi.org/10.1007/s10522-014-9536-x.
During A, Penel G, Hardouin P. Understanding the local actions of lipids in bone physiology. Prog Lipid Res. 2015;59:126–46. https://doi.org/10.1016/j.plipres.2015.06.002.
Elbaz A, Wu X, Rivas D, Gimble JM, Duque G. Inhibition of fatty acid biosynthesis prevents adipocyte lipotoxicity on human osteoblasts in vitro. J Cell Mol Med. 2010;14(4):982–91. https://doi.org/10.1111/j.1582-4934.2009.00751.x.
Jiang X, Song D, Ye B, Wang X, Song G, Yang S, et al. Effect of intermittent administration of adiponectin on bone regeneration following mandibular osteodistraction in rabbits. J Orthop Res. 2011;29(7):1081–5. https://doi.org/10.1002/jor.21355.
Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 2013;17(6):901–15. https://doi.org/10.1016/j.cmet.2013.04.009.
Naot D, Watson M, Callon KE, Tuari D, Musson DS, Choi AJ, et al. Reduced bone density and cortical bone indices in female adiponectin-knockout mice. Endocrinology. 2016;157(9):3550–61. https://doi.org/10.1210/en.2016-1059.
Naot D, Musson DS, Cornish J. The activity of adiponectin in bone. Calcif Tissue Int. 2017;100(5):486–99. https://doi.org/10.1007/s00223-016-0216-5.
Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63. https://doi.org/10.1038/nature08099.
Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, et al. Bone marrow and the control of immunity. Cell Mol Immunol. 2012;9(1):11–9. https://doi.org/10.1038/cmi.2011.47.
Busso N, So A, Chobaz-Peclat V, Morard C, Martinez-Soria E, Talabot-Ayer D, et al. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J Immunol. 2002;168(2):875–82.
•• Adler BJ, Green DE, Pagnotti GM, Chan ME, Rubin CT. High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLOS ONE. 2014;9(3):e90639. https://doi.org/10.1371/journal.pone.0090639. The results of this study indicate that obesity is a high risk factor for the defective leukogenesis of B cells by suppressing the expression of Il-7.
Bonomo A, Monteiro AC, Goncalves-Silva T, Cordeiro-Spinetti E, Galvani RG, Balduino A. A T Cell View Of the bone marrow. Front Immunol. 2016;7:184. https://doi.org/10.3389/fimmu.2016.00184.
Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–48. https://doi.org/10.1182/blood-2006-07-037994.
Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, et al. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A. 2001;98(24):13960–5. https://doi.org/10.1073/pnas.251534698.
McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, et al. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 2014;34(12):2637–43. https://doi.org/10.1161/ATVBAHA.114.304636.
Gruver AL, Ventevogel MS, Sempowski GD. Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution. J Endocrinol. 2009;203(1):75–85. https://doi.org/10.1677/JOE-09-0179.
Matarese G. Leptin and the immune system: how nutritional status influences the immune response. Eur Cytokine Netw. 2000;11(1):7–14.
Ioan-Facsinay A, Kwekkeboom JC, Westhoff S, Giera M, Rombouts Y, van Harmelen V, et al. Adipocyte-derived lipids modulate CD4+ T-cell function. Eur J Immunol. 2013;43(6):1578–87. https://doi.org/10.1002/eji.201243096.
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88. https://doi.org/10.1038/nm.2279.
D'Amico L, Roato I. Cross-talk between T cells and osteoclasts in bone resorption. Bonekey Rep. 2012;1:82. https://doi.org/10.1038/bonekey.2012.82.
Liu LF, Shen WJ, Ueno M, Patel S, Kraemer FB. Characterization of age-related gene expression profiling in bone marrow and epididymal adipocytes. BMC Genomics. 2011;12:212. https://doi.org/10.1186/1471-2164-12-212.
Dib LH, Ortega MT, Fleming SD, Chapes SK, Melgarejo T. Bone marrow leptin signaling mediates obesity-associated adipose tissue inflammation in male mice. Endocrinology. 2014;155(1):40–6. https://doi.org/10.1210/en.2013-1607.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AJ. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. https://doi.org/10.1172/JCI19246.
Lee BC, Kim MS, Pae M, Yamamoto Y, Eberle D, Shimada T, et al. Adipose natural killer cells regulate adipose tissue macrophages to promote insulin resistance in obesity. Cell Metab. 2016;23(4):685–98. https://doi.org/10.1016/j.cmet.2016.03.002.
Gong L, Zhao Y, Zhang Y, Ruan Z. The macrophage polarization regulates MSC osteoblast differentiation in vitro. Ann Clin Lab Sci. 2016;46(1):65–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jillian Cornish, Tao Wang, and Jian-Ming Lin declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Bone Marrow and Adipose Tissue
Rights and permissions
About this article
Cite this article
Cornish, J., Wang, T. & Lin, Jm. Role of Marrow Adipocytes in Regulation of Energy Metabolism and Bone Homeostasis. Curr Osteoporos Rep 16, 116–122 (2018). https://doi.org/10.1007/s11914-018-0425-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0425-0