Abstract
Purpose of Review
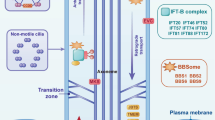
The primary cilium is a non-motile microtubule-based organelle that senses a diverse range of extracellular signals. While recent studies highlight the importance of ciliary-dependent developmental signals, including Hedgehog, Wnt, and platelet-derived growth factor, it is not well understood whether and how bone morphogenetic protein (BMP) signaling, a key regulator of skeletogenesis, is involved in cilia-related bone developmental aspects and in the etiology of skeletal disorders.
Recent Findings
Increasing evidence suggests that osteoblast- or osteocyte-specific deletion of ciliary proteins leads to diverse skeletal malformations, reinforcing the idea that primary cilia are indispensable for regulating bone development and maintenance. Furthermore, it became evident that ciliary proteins not only contribute to ciliogenesis but also orchestrate cellular trafficking.
Summary
This review summarizes the current understanding of ciliary proteins in bone development and discusses the potential role of BMP signaling in primary cilia, enabling us to unravel the potential pathogenesis of skeletal ciliopathies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •Of importance••Of major importance
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38.
Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30(1):2–4.
Santos N, Reiter JF. Building it up and taking it down: the regulation of vertebrate ciliogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237(8):1972–81.
Sanchez I, Dynlacht BD. Cilium assembly and disassembly. Nat Cell Biol. 2016;18(7):711–7.
Oh EC, Katsanis N. Cilia in vertebrate development and disease. Development. 2012;139(3):443–8.
Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23(1):345–73.
Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19(13):R526–35.
Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MP, et al. TGF-beta signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 2013;3(6):1806–14.
Mourao A, Christensen ST, Lorentzen E. The intraflagellar transport machinery in ciliary signaling. Curr Opin Struct Biol. 2016;41:98–108.
Yuan X, Serra RA, Yang S. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann N Y Acad Sci. 2015;1335(1):78–99.
Novarino G, Akizu N, Gleeson JG. Modeling human disease in humans: the ciliopathies. Cell. 2011;147(1):70–9.
Yuan X, Yang S. Cilia/Ift protein and motor-related bone diseases and mouse models. Frontiers in bioscience (Landmark edition). 2015;20:515–55.
Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA-synthesis in 3t3-cells. Cell. 1979;17(3):527–35.
Kobayashi T, Dynlacht BD. Regulating the transition from centriole to basal body. J Cell Biol. 2011;193(3):435–44.
Nigg EA, Stearns T. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol. 2011;13(10):1154–60.
Gupta GD, Coyaud E, Goncalves J, Mojarad BA, Liu Y, Wu Q, et al. A dynamic protein interaction landscape of the human centrosome-cilium interface. Cell. 2015;163(6):1484–99.
Conduit PT, Wainman A, Raff JW. Centrosome function and assembly in animal cells. Nat Rev Mol Cell Biol. 2015;16(10):611–24.
Tonna EA, Lampen NM. Electron microscopy of aging skeletal cells. I. Centrioles and solitary cilia. J Gerontol. 1972;27(3):316–24.
Matthews JL, Martin JH. Intracellular transport of calcium and its relationship to homeostasis and mineralization: an electron microscope study. Am J Med. 1971;50(5):589–97.
Whitfield JF. Primary cilium—is it an osteocyte’s strain-sensing flowmeter? J Cell Biochem. 2003;89(2):233–7.
• Coughlin TR, Voisin M, Schaffler MB, Niebur GL, McNamara LM. Primary cilia exist in a small fraction of cells in trabecular bone and marrow. Calcif Tissue Int. 2015;96(1):65–72. This study demonstrated that only 4% of osteoblasts/osteocytes express primary cilia, indicating that cilia may act in a selected cell population
Uzbekov RE, Maurel DB, Aveline PC, Pallu S, Benhamou CL, Rochefort GY. Centrosome fine ultrastructure of the osteocyte mechanosensitive primary cilium. Microsc Microanal. 2012;18(6):1430–41.
Xiao Z, Dallas M, Qiu N, Nicolella D, Cao L, Johnson M, et al. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25(7):2418–32.
Tummala P, Arnsdorf EJ, Jacobs CR. The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell Mol Bioeng. 2010;3(3):207–12.
Hoey DA, Tormey S, Ramcharan S, O'Brien FJ, Jacobs CR. Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells. 2012;30(11):2561–70.
Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, et al. Origin of osteoclasts—mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(18):7260–4.
Finetti F, Paccani SR, Rosenbaum J, Baldari CT. Intraflagellar transport: a new player at the immune synapse. Trends Immunol. 2011;32(4):139–45.
• Singh M, Chaudhry P, Ramsingh G, Merchant A. Presence of primary cilia in human hematopoietic system. Blood. 2015;126(23):4762. This study demonstrated the presence of primary cilia in the hematopoietic system for the first time. The results implicate loss of primary cilia in the development of hematological malignancies through deregulated Hh signaling
Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(33):13325–30.
Qiu N, Cao L, David V, Quarles LD, Xiao Z. Kif3a deficiency reverses the skeletal abnormalities in Pkd1 deficient mice by restoring the balance between osteogenesis and adipogenesis. PLoS One. 2010;5(12):e15240.
Xiao Z, Zhang S, Cao L, Qiu N, David V, Quarles LD. Conditional disruption of Pkd1 in osteoblasts results in osteopenia due to direct impairment of bone formation. J Biol Chem. 2010;285(2):1177–87.
Qiu N, Xiao Z, Cao L, Buechel MM, David V, Roan E, et al. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J Cell Sci. 2012;125(Pt 8):1945–57.
•• Yuan X, Cao J, He X, Serra R, Qu J, Cao X, et al. Ciliary IFT80 balances canonical versus non-canonical hedgehog signalling for osteoblast differentiation. Nat Commun. 2016;7:11024. This study revealed that IFT80 is required for osteoblast differentiation by balancing the canonical Hh-Gli and non-canonical Hh-Galphai-RhoA pathways
• Chen JC, Hoey DA, Chua M, Bellon R, Jacobs CR. Mechanical signals promote osteogenic fate through a primary cilia-mediated mechanism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30(4):1504–11. This study demonstrated that mechanical loading stimulates the homing of bone marrow cells to the bone surface and increases bone mass. In response to mechanical loading, deletion of Kif3a in the recruited osteoblasts significantly decreases the amount of bone formation
Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, et al. Primary cilia are not calcium-responsive mechanosensors. Nature. 2016;531(7596):656–60.
Lindbæk L, Warzecha CB, Koefoed K, Mogensen JB, Schmid F, Pedersen LB, et al. Coordination of TGFβ/BMP signaling is associated with the primary cilium. Cilia. 2015;4(Suppl 1):P17.
Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9(1):49–61.
Ehrlich M. Endocytosis and trafficking of BMP receptors: regulatory mechanisms for fine-tuning the signaling response in different cellular contexts. Cytokine Growth Factor Rev. 2016;27:35–42.
Komatsu Y, Kaartinen V, Mishina Y. Cell cycle arrest in node cells governs ciliogenesis at the node to break left-right symmetry. Development. 2011;138(18):3915–20.
Franzen A, Heldin NE. BMP-7-induced cell cycle arrest of anaplastic thyroid carcinoma cells via p21(CIP1) and p27(KIP1). Biochem Biophys Res Commun. 2001;285(3):773–81.
Alcalay NI, Sharma M, Vassmer D, Chapman B, Paul B, Zhou J, et al. Acceleration of polycystic kidney disease progression in cpk mice carrying a deletion in the homeodomain protein Cux1. Am J Physiol Renal Physiol. 2008;295(6):F1725–34.
Cadieux C, Harada R, Paquet M, Cote O, Trudel M, Nepveu A, et al. Polycystic kidneys caused by sustained expression of Cux1 isoform p75. J Biol Chem. 2008;283(20):13817–24.
Thomas DM, Johnson SA, Sims NA, Trivett MK, Slavin JL, Rubin BP, et al. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol. 2004;167(5):925–34.
Chang SF, Chang TK, Peng HH, Yeh YT, Lee DY, Yeh CR, et al. BMP-4 induction of arrest and differentiation of osteoblast-like cells via p21 CIP1 and p27 KIP1 regulation. Mol Endocrinol. 2009;23(11):1827–38.
Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272–88.
Wu M, Chen G, Li Y-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Research. 2016;4:16009.
Sanchez-Duffhues G, de Vinuesa AG, Lindeman JH, Mulder-Stapel A, DeRuiter MC, Van Munsteren C, et al. SLUG is expressed in endothelial cells lacking primary cilia to promote cellular calcification. Arterioscler Thromb Vasc Biol. 2015;35(3):616–27.
• Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1(1):87–105. This study demonstrated that non-ciliated aortic endothelial cells acquire the ability to transdifferentiate into osteogenic cells in a BMP-dependent manner, and that β-catenin–induced Slug is a key transcription factor controlling this process
Vertii A, Bright A, Delaval B, Hehnly H, Doxsey S. New frontiers: discovering cilia-independent functions of cilia proteins. EMBO Rep. 2015;16(10):1275–87.
•• Noda K, Kitami M, Kitami K, Kaku M, Komatsu Y. Canonical and noncanonical intraflagellar transport regulates craniofacial skeletal development. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(19):E2589-97. This study demonstrated that neural crest-specific deletion of IFT20 compromises not only ciliogenesis but also intracellular transport of collagen, which leads to osteopenia in the facial region.
Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol. 2009;11(11):1332–U163.
Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17(9):3781–92.
Baldari CT, Rosenbaum J. Intraflagellar transport: it’s not just for cilia anymore. Curr Opin Cell Biol. 2010;22(1):75–80.
Finetti F, Paccani SR, Rosenbaum J, Baldari CT. Intraflagellar transport: a new player at the immune synapse. Trends Immunol. 2011;32(4):139–45.
Ocbina PJ, Eggenschwiler JT, Moskowitz I, Anderson KV. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet. 2011;43(6):547–53.
Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-beta-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J Cell Sci. 2012;125(Pt 5):1259–73.
Hart EK, Jinnin M, Hou B, Fukai N, Olsen BR. Kinesin-2 controls development and patterning of the vertebrate skeleton by Hedgehog-and Gli3-dependent mechanisms. Dev Biol. 2007;309(2):273–84.
Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, et al. Intraflagellar transport is essential for endochondral bone formation. Development. 2007;134(2):307–16.
Liu B, Chen S, Cheng D, Jing W, Helms JA. Primary cilia integrate hedgehog and Wnt signaling during tooth development. J Dent Res. 2014;93(5):475–82.
Temiyasathit S, Tang WJ, Leucht P, Anderson CT, Monica SD, Castillo AB, et al. Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PLoS One. 2012;7(3):e33368.
Yuan X, Yang S. Primary cilia and intraflagellar transport proteins in bone and cartilage. J Dent Res. 2016;95(12):1341–9.
Acknowledgements
We gratefully acknowledge Patricia Fonseca for editorial assistance. This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (26293407, 15KK0337, and 15K15704 to MK), and by a grant from the NIDCR/NIH (R00DE021054 to YK).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Masaru Kaku and Yoshihiro Komatsu declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Skeletal Development
Rights and permissions
About this article
Cite this article
Kaku, M., Komatsu, Y. Functional Diversity of Ciliary Proteins in Bone Development and Disease. Curr Osteoporos Rep 15, 96–102 (2017). https://doi.org/10.1007/s11914-017-0351-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-017-0351-6