Abstract
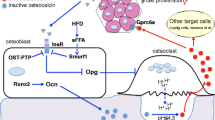
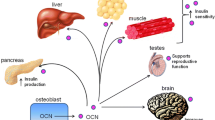
A recent unexpected development of bone biology is that bone is an endocrine organ regulating a growing number of physiological processes. One of the functions regulated by bone through the hormone osteocalcin is glucose homeostasis. In this overview, we will explain why we hypothesized that bone mass and energy metabolism should be subjected to a coordinated endocrine regulation. We will then review the experiments that revealed the endocrine function of osteocalcin and the cell biology events that allow osteocalcin to become a hormone. We will also illustrate the importance of this regulation to understand whole-body glucose homeostasis in the physiological state and in pathological conditions. Lastly, we will mention epidemiological and genetic evidence demonstrating that this function of osteocalcin is conserved in humans.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major importance
Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–14.
Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–48.
Mika C, Holtkamp K, Heer M, Gunther RW, Herpertz-Dahlmann B. A 2-year prospective study of bone metabolism and bone mineral density in adolescents with anorexia nervosa. J Neural Transm. 2007;114:1611–8.
Audi L, Vargas DM, Gussinye M, Yeste D, Marti G, Carrascosa A. Clinical and biochemical determinants of bone metabolism and bone mass in adolescent female patients with anorexia nervosa. Pediatr Res. 2002;51:497–504.
Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–96.
Jacoangeli F, Zoli A, Taranto A, Staar Mezzasalma F, Ficoneri C, Pierangeli S, et al. Osteoporosis and anorexia nervosa: relative role of endocrine alterations and malnutrition. Eating Weight Disorders: EWD. 2002;7:190–5.
Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–23.
Misra M, Katzman DK, Cord J, Manning SJ, Mendes N, Herzog DB, et al. Bone metabolism in adolescent boys with anorexia nervosa. J Clin Endocrinol Metab. 2008;93:3029–36.
Misra M, Klibanski A. Bone metabolism in adolescents with anorexia nervosa. J Endocrinol Investig. 2011;34:324–32.
Misra M, Klibanski A. Anorexia nervosa, obesity and bone metabolism. Pediatric Endocrinol Rev: PER. 2013;11:21–33.
Fazeli PK, Klibanski A. Bone metabolism in anorexia nervosa. Curr Osteoporosis Reports. 2014;12:82–9.
Himes JH. Bone growth and development in protein-calorie malnutrition. World Rev Nutr Diet. 1978;28:143–87.
Faridi MM, Ansari Z, Bhargava SK. Imprints of protein energy malnutrition on the skeleton of children. J Trop Pediatr. 1984;30:150–3.
Hauschka PV, Lian JB, Gallop PM. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975;72:3925–9.
Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976;73:1447–51.
Price PA, Poser JW, Raman N. Primary structure of the gamma-carboxyglutamic acid-containing protein from bovine bone. Proc Natl Acad Sci U S A. 1976;73:3374–5.
Desbois C, Hogue DA, Karsenty G. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biological Chem. 1994;269:1183–90.
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–52.
Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–30.
Mauro LJ, Olmsted EA, Skrobacz BM, Mourey RJ, Davis AR, Dixon JE. Identification of a hormonally regulated protein tyrosine phosphatase associated with bone and testicular differentiation. J Biol Chem. 1994;269:30659–67.
Morrison DF, Mauro LJ. Structural characterization and chromosomal localization of the mouse cDNA and gene encoding the bone tyrosine phosphatase, mOST-PTP. Gene. 2000;257:195–208.
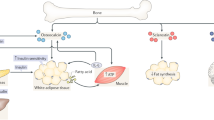
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. This is an original study revealing the physiological function of osteocalcin in regulating glucose metabolism.
Poser JW, Esch FS, Ling NC, Price PA. Isolation and sequence of the vitamin K-dependent protein from human bone. Undercarboxylation of the first glutamic acid residue. J Biol Chem. 1980;255:8685–91.
Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–70. This study demonstrated the direct physiological functions of osteocalcin toward pancreatic beta cells and adipocytes in WT mice.
Ferron M, Wei J, Yoshizawa T, Ducy P, Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem Biophys Res Commun. 2010;397:691–6.
Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. This study uncovered that insulin signaling in osteoblasts is necessary for whole-body glucose homeostasis by favoring bone resorption to activate osteocalcin.
Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–19.
Wei J, Ferron M, Clarke CJ, Hannun YA, Jiang H, Blaner WS, et al. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest. 2014;124:1–13. This study explored the pathogenetic contribution of the local insulin resistance in bone to the high fat diet induced insulin resistance and identified a molecular mechanism causing the bone specific insulin resistance.
Im JA, Yu BP, Jeon JY, Kim SH. Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clinica Chimica Acta; Int J Clin Chem. 2008;396:66–9.
Hwang YC, Jeong IK, Ahn KJ, Chung HY. The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta-cell function in middle-aged male subjects. Diabetes Metab Res Rev. 2009;25:768–72.
Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:45–9.
Kindblom JM, Ohlsson C, Ljunggren O, Karlsson MK, Tivesten A, Smith U, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J bone Min Res: Off J Am Soc Bone Min Res. 2009;24:785–91.
Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, et al. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol/Eur Federation Endocrine Soc. 2009;161:723–9.
Hwang, Y.C., Jeong, I.K., Ahn, K.J., and Chung, H.Y. Circulating osteocalcin level is associated with improved glucose tolerance, insulin secretion and sensitivity independent of the plasma adiponectin level. Osteoporos Int. 2012;23:1337–42. doi:10.1007/s00198-011-1679-x.
Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, et al. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22:187–94.
Strapazzon G, De Toni L, Foresta C. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporosis Int: J Established Result Cooperation Between Eur Foundation Osteoporosis National Osteoporosis Foundation USA. 2011;22:1643–4.
Wedrychowicz A, Stec M, Sztefko K, Starzyk JB. Associations between bone, fat tissue and metabolic control in children and adolescents with type 1 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122:491–5.
Levinger, I., Jerums, G., Stepto, N.K., Parker, L., Serpiello, F.R., McConell, G.K., Anderson, M., Hare, D.L., Byrnes, E., Ebeling, P.R., et al. (2014). The effect of acute exercise on undercarboxylated osteocalcin and insulin sensitivity in obese men. J Bone Miner Res. 2014;29:2571–6. doi:10.1002/jbmr.2285.
Kim GS, Jekal Y, Kim HS, Im JA, Park JY, Chu SH. Reduced serum total osteocalcin is associated with central obesity in Korean children. Obesity Res Clin Pract. 2014;8:e201–298.
Garanty-Bogacka B, Syrenicz M, Rac M, Krupa B, Czaja-Bulsa G, Walczak M, et al. Association between serum osteocalcin, adiposity and metabolic risk in obese children and adolescents. Endokrynologia Polska. 2013;64:346–52.
Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011;144:796–809. This study identified Gprc6a as a osteocalcin receptor.
Wellendorph P, Brauner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46.
Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005;280:40201–9.
Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes beta-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014;63:1021–31. This study identified Gprc6a as a receptor mediating osteocalin functions in pancreatic beta cells.
Boisen KA, Main KM, Rajpert-De Meyts E, Skakkebaek NE. Are male reproductive disorders a common entity? The testicular dysgenesis syndrome. Ann N Y Acad Sci. 2001;948:90–9.
Glass AR, Vigersky RA. Testicular reserve of testosterone precursors in primary testicular failure. Fertil Steril. 1982;38:92–6.
Paduch DA. Testicular cancer and male infertility. Curr Opin Urol. 2006;16:419–27.
Winters SJ, Troen P. A reexamination of pulsatile luteinizing hormone secretion in primary testicular failure. J Clin Endocrinol Metab. 1983;57:432–5.
Oury F, Ferron M, Huizhen W, Confavreux C, Xu L, Lacombe J, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123:2421–33. This study reported that two human subjects with mutations in Gprc6a, a receptor for osteocalcin, demonstrated similar abnormalities in fertility as described in mice lack of osteocalcin.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
J Wei and G Karsenty both declare no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Bone and Diabetes
Rights and permissions
About this article
Cite this article
Wei, J., Karsenty, G. An Overview of the Metabolic Functions of Osteocalcin. Curr Osteoporos Rep 13, 180–185 (2015). https://doi.org/10.1007/s11914-015-0267-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-015-0267-y