Abstract
Purpose of Review
Anaplastic thyroid carcinoma is a type of thyroid carcinoma with the most aggressive biological behaviour amongst thyroid cancer. Here, we review the current genomic and the impacts of advances in therapies to improve the management of patients with the cancer.
Recent Findings
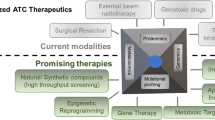
Common mutations being identified in anaplastic thyroid carcinoma are p53 and TERT promoter mutations. Other common mutated genes included BRAF, RAS, EIF1AX, PIK3CA, PTEN and AKT1, SWI/SNF, ALK and CDKN2A. Changes in expression of different microRNAs are also involved in the pathogenesis of anaplastic thyroid carcinoma. Curative resection combined with radiotherapy and combination chemotherapies (such as anthracyclines, platins and taxanes) has been shown to have effects in the treatment of some patients with anaplastic thyroid carcinoma. Newer molecular targeted therapies in clinical trials target mostly the cell membrane kinase and downstream proteins. These include targeting the EGFR, FGFR, VEGFR, c-kit, PDGFR and RET on the cell membrane as well as VEGF itself and the downstream targets such as BRAF, MEK and mTOR. Immunotherapy is also being tested in the cancer.
Summary
Updated knowledge of genomic as well as clinical trials on novel therapies is needed to improve the management of the patients with this aggressive cancer.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Lam KY, Lo CY, Chan KW, Wan KY. Insular and anaplastic carcinoma of the thyroid: a 45-year comparative study at a single institution and a review of the significance of p53 and p21. Ann Surg. 2000;231:329–38. https://doi.org/10.1097/00000658-200003000-00005.
• Yoo SK, Song YS, Lee EK, Hwang J, Kim HH, Jung G, et al. Integrative analysis of genomic and transcriptomic characteristics associated with progression of aggressive thyroid cancer. Nat Commun. 2019;10:2764. https://doi.org/10.1038/s41467-019-10680-5. This study performed whole-genome sequencing in patients with anaplastic thyroid carcinoma.
Kunstman JW, Juhlin CC, Goh G, Brown TC, Stenman A, Healy JM, et al. Characterisation of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet. 2015;24:2318–29. https://doi.org/10.1093/hmg/ddu749.
Ravi N, Yang M, Gretarsson S, Jansson C, Mylona N, Sydow SR, et al. Identification of Targetable Lesions in Anaplastic Thyroid Cancer by Genome Profiling. Cancers (Basel). 2019;11:402. https://doi.org/10.3390/cancers11030402.
Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin Cancer Res. 2018;24:3059–68. https://doi.org/10.1158/1078-0432.CCR-18-0373.
Latteyer S, Tiedje V, König K, Ting S, Heukamp LC, Meder L, et al. Targeted next-generation sequencing for TP53, RAS, BRAF, ALK and NF1 mutations in anaplastic thyroid cancer. Endocrine. 2016;54:733–41. https://doi.org/10.1007/s12020-016-1080-9.
Khan SA, Ci B, Xie Y, Gerber DE, Beg MS, Sherman SI, et al. Unique mutation patterns in anaplastic thyroid cancer identified by comprehensive genomic profiling. Head Neck. 2019;41:1928–34. https://doi.org/10.1002/hed.25634.
Jeon MJ, Chun SM, Kim D, Kwon H, Jang EK, Kim TY, et al. Genomic Alterations of Anaplastic Thyroid Carcinoma Detected by Targeted Massive Parallel Sequencing in a BRAF(V600E) Mutation-Prevalent Area. Thyroid. 2016;26:683–90. https://doi.org/10.1089/thy.2015.0506.
Duan H, Li Y, Hu P, Gao J, Ying J, Xu W, et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology. 2019;75:890–9. https://doi.org/10.1111/his.13942.
Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126:1052–66. https://doi.org/10.1172/JCI85271.
Hanna GJ, Busaidy NL, Chau NG, Wirth LJ, Barletta JA, Calles A, et al. Genomic Correlates of Response to Everolimus in Aggressive Radioiodine-refractory Thyroid Cancer: A Phase II Study. Clin Cancer Res. 2018;24:1546–53. https://doi.org/10.1158/1078-0432.CCR-17-2297.
Tiedje V, Ting S, Herold T, Synoracki S, Latteyer S, Moeller LC, et al. NGS based identification of mutational hotspots for targeted therapy in anaplastic thyroid carcinoma. Oncotarget. 2017;8:42613–20. https://doi.org/10.18632/oncotarget.17300.
Deeken-Draisey A, Yang GY, Gao J, Alexiev BA. Anaplastic thyroid carcinoma: an epidemiologic, histologic, immunohistochemical, and molecular single-institution study. Hum Pathol. 2018;82:140–8. https://doi.org/10.1016/j.humpath.2018.07.027.
Bonhomme B, Godbert Y, Perot G, Al Ghuzlan A, Bardet S, Belleannée G, et al. Molecular Pathology of Anaplastic Thyroid Carcinomas: A Retrospective Study of 144 Cases. Thyroid. 2017;27:682–92. https://doi.org/10.1089/thy.2016.0254.
Lam KY, Tsao SW, Zhang D, Law S, He D, Ma L, et al. Prevalence and predictive value of p53 mutation in patients with oesophageal squamous cell carcinomas: a prospective clinico-pathological study and survival analysis of 70 patients. Int J Cancer. 1997;74:212–9. https://doi.org/10.1002/(sici)1097-0215(19970422)74:2<212::aid-ijc13>3.0.co;2-f.
Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–58. https://doi.org/10.1038/nrc2723.
Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44. https://doi.org/10.1677/ERC-08-0154.
Perri F, Pisconti S, Della Vittoria Scarpati G. P53 mutations and cancer: a tight linkage. Ann Transl Med. 2016;4:522. https://doi.org/10.21037/atm.2016.12.40.
Panebianco F, Nikitski AV, Nikiforova MN, Nikiforov YE. Spectrum of TERT promoter mutations and mechanisms of activation in thyroid cancer. Cancer Med. 2019;8:5831–9. https://doi.org/10.1002/cam4.2467.
Melo M, da Rocha AG, Vinagre J, Batista R, Peixoto J, Tavares C, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014;99:E754–65. https://doi.org/10.1210/jc.2013-3734.
Shi X, Liu R, Qu S, Zhu G, Bishop J, Liu X, et al. Association of TERT promoter mutation 1,295,228 C>T with BRAF V600E mutation, older patient age, and distant metastasis in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2015;100:E632–7. https://doi.org/10.1210/jc.2014-3606.
Salajegheh A, Petcu EB, Smith RA, Lam AK. Follicular variant of papillary thyroid carcinoma: a diagnostic challenge for clinicians and pathologists. Postgrad Med J. 2008;84:78–82. https://doi.org/10.1136/pgmj.2007.064881.
Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016;12:e1006239. https://doi.org/10.1371/journal.pgen.1006239.
Karoulia Z, Gavathiotis E, Poulikakos PI. New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer. 2017;17:676–91. https://doi.org/10.1038/nrc.2017.79.
Dankner M, Rose AAN, Rajkumar S, Siegel PM, Watson IR. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene. 2018;37:3183–99. https://doi.org/10.1038/s41388-018-0171-x.
Ng JY, Lu CT, Lam AK. BRAF mutation: Current and future clinical pathological applications in colorectal carcinoma. Histol Histopathol. 2019;34:469–77. https://doi.org/10.14670/HH-18-079.
Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346–56. https://doi.org/10.1097/PAT.0b013e328360b61d.
Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med. 2013;368:684–5. https://doi.org/10.1056/NEJMc1215697.
Rushton S, Burghel G, Wallace A, Nonaka D. Immunohistochemical detection of BRAF V600E mutation status in anaplastic thyroid carcinoma. Histopathology. 2016;69:524–6. https://doi.org/10.1111/his.12964.
Smith RA, Salajegheh A, Weinstein S, Nassiri M, Lam AK. Correlation between BRAF mutation and the clinicopathological parameters in papillary thyroid carcinoma with particular reference to follicular variant. Hum Pathol. 2011;42:500–6. https://doi.org/10.1016/j.humpath.2009.09.023.
Rahman MA, Salajegheh A, Smith RA, Lam AK. Multiple proliferation-survival signalling pathways are simultaneously active in BRAF V600E mutated thyroid carcinomas. Exp Mol Pathol. 2015;99:492–7. https://doi.org/10.1016/j.yexmp.2015.09.006.
Huang Y, Qu S, Zhu G, Wang F, Liu R, Shen X, et al. BRAF V600E mutation-assisted risk stratification of solitary intrathyroidal papillary thyroid cancer for precision treatment. J Natl Cancer Inst. 2018;110:362–70. https://doi.org/10.1093/jnci/djx227.
Kim KJ, Kim SG, Tan J, Shen X, Viola D, Elisei R, et al. BRAF V600E status may facilitate decision-making on active surveillance of low-risk papillary thyroid microcarcinoma. Eur J Cancer. 2020;124:161–9. https://doi.org/10.1016/j.ejca.2019.10.017.
Shen X, Zhu G, Liu R, Viola D, Elisei R, Puxeddu E, et al. Patient Age-associated mortality risk is differentiated by BRAF V600E status in papillary thyroid cancer. J Clin Oncol. 2018;36:438–45. https://doi.org/10.1200/JCO.2017.74.5497.
Wang F, Zhao S, Shen X, Zhu G, Liu R, Viola D, et al. BRAF V600E confers male sex disease-specific mortality risk in patients with papillary thyroid cancer. J Clin Oncol. 2018;36:2787–95. https://doi.org/10.1200/JCO.2018.78.5097.
Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. J Am Med Assoc. 2013;309:1493–501. https://doi.org/10.1001/jama.2013.3190.
Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33:42–50. https://doi.org/10.1200/JCO.2014.56.8253.
Asati V, Bharti SK, Mahapatra DK, Asati V, Budhwani AK. Triggering PIK3CA Mutations in PI3K/Akt/mTOR Axis: Exploration of Newer Inhibitors and Rational Preventive Strategies. Curr Pharm Des. 2016;22:6039–54. https://doi.org/10.2174/1381612822666160614000053.
Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15:7–24. https://doi.org/10.1038/nrc3860.
Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455–9. https://doi.org/10.1038/sj.bjc.6602970.
Janku F, Lee JJ, Tsimberidou AM, Hong DS, Naing A, Falchook GS, et al. PIK3CA mutations frequently coexist with RAS and BRAF mutations in patients with advanced cancers. PLoS One. 2011;6:e22769. https://doi.org/10.1371/journal.pone.0022769.
Jin J, Shi Y, Zhang S, Yang S. PIK3CA mutation and clinicopathological features of colorectal cancer: a systematic review and Meta-Analysis. Acta Oncol. 2020;59:66–74. https://doi.org/10.1080/0284186X.2019.1664764.
Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–96. https://doi.org/10.1038/nrm3330.
Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–44. https://doi.org/10.1038/nature05933.
Lu C, Allis CD. SWI/SNF complex in cancer. Nat Genet. 2017;49:178–9. https://doi.org/10.1038/ng.3779.
Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene. 2009;28:1653–68. https://doi.org/10.1038/onc.2009.4.
Tennstedt P, Strobel G, Bölch C, Grob T, Minner S, Masser S, et al. Patterns of ALK expression in different human cancer types. J Clin Pathol. 2014;67:477–81. https://doi.org/10.1136/jclinpath-2013-201991.
Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer. 2018;9:423–30. https://doi.org/10.1111/1759-7714.12613.
Devarakonda S, Morgensztern D, Govindan R. Genomic alterations in lung adenocarcinoma. Lancet Oncol. 2015;16:e342–51. https://doi.org/10.1016/S1470-2045(15)00077-7.
Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–16. https://doi.org/10.1210/jc.2008-0273.
Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23:1988–97. https://doi.org/10.1158/1078-0432.CCR-16-1679.
Gopalan V, Islam F, Pillai S, Tang JC, Tong DK, Law S, et al. Overexpression of microRNA-1288 in oesophageal squamous cell carcinoma. Exp Cell Res. 2016;348:146–54. https://doi.org/10.1016/j.yexcr.2016.09.010.
Lee KT, Tan JK, Lam AK, Gan SY. MicroRNAs serving as potential biomarkers and therapeutic targets in nasopharyngeal carcinoma: A critical review. Crit Rev Oncol Hematol. 2016;103:1–9. https://doi.org/10.1016/j.critrevonc.2016.04.006.
Islam F, Gopalan V, Vider J, Wahab R, Ebrahimi F, Lu CT, et al. MicroRNA-186-5p overexpression modulates colon cancer growth by repressing the expression of the FAM134B tumour inhibitor. Exp Cell Res. 2017;357:260–70. https://doi.org/10.1016/j.yexcr.2017.05.021.
Mamoori A, Gopalan V, Lam AK. Role of miR-193a in cancer: complexity and factors control the pattern of its expression. Curr Cancer Drug Targets. 2018;18:618–28. https://doi.org/10.2174/1568009618666180308105727.
Amin M, Islam F, Gopalan V, Lam AK. Detection and quantification of MicroRNAs in esophageal adenocarcinoma. Methods Mol Biol. 1756;2018:257–68. https://doi.org/10.1007/978-1-4939-7734-5_22.
Gopalan V, Ebrahimi F, Islam F, Vider J, Qallandar OB, Pillai S, et al. Tumour suppressor properties of miR-15a and its regulatory effects on BCL2 and SOX2 proteins in colorectal carcinomas. Exp Cell Res. 2018;370:245–53. https://doi.org/10.1016/j.yexcr.2018.06.025.
Han L, Cui D, Li B, Xu WW, Lam AKY, Chan KT, et al. MicroRNA-338-5p reverses chemoresistance and inhibits invasion of esophageal squamous cell carcinoma cells by targeting Id-1. Cancer Sci. 2019;110:3677–88. https://doi.org/10.1111/cas.14220.
Mamoori A, Wahab R, Vider J, Gopalan V, Lam AK. The tumour suppressor effects and regulation of cancer stem cells by macrophage migration inhibitory factor targeted miR-451 in colon cancer. Gene. 2019;697:165–74. https://doi.org/10.1016/j.gene.2019.02.046.
Islam F, Gopalan V, Lam AK. Roles of MicroRNAs in esophageal squamous cell carcinoma pathogenesis. Methods Mol Biol. 2020;2129:241–57. https://doi.org/10.1007/978-1-0716-0377-2_18.
Luo Y, Xiong W, Dong S, Liu F, Liu H, Li J. MicroRNA-146a promotes the proliferation of rat vascular smooth muscle cells by downregulating p53 signaling. Mol Med Rep. 2017;16:6940–5. https://doi.org/10.3892/mmr.2017.7477.
Shen C, Yang H, Liu H, Wang X, Zhang Y, Xu R. Inhibitory effect and mechanisms of microRNA-146b-5p on the proliferation and metastatic potential of Caski human cervical cancer cells. Mol Med Rep. 2015;11:3955–61. https://doi.org/10.3892/mmr.2015.3151.
Ramírez-Moya J, Wert-Lamas L, Santisteban P. MicroRNA-146b promotes PI3K/AKT pathway hyperactivation and thyroid cancer progression by targeting PTEN. Oncogene. 2018;37:3369–83. https://doi.org/10.1038/s41388-017-0088-9.
Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600–8. https://doi.org/10.1210/jc.2007-2696.
Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V, et al. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer. 2007;14:791–8. https://doi.org/10.1677/ERC-07-0129.
Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13:317–23. https://doi.org/10.1038/ncb2173.
Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X, et al. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy. 2009;5:816–23. https://doi.org/10.4161/auto.9064.
Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47. https://doi.org/10.1016/j.cell.2005.01.014.
Maroof H, Irani S, Arianna A, Vider J, Gopalan V, Lam AK. Interactions of Vascular Endothelial Growth Factor and p53 with miR-195 in Thyroid Carcinoma: Possible Therapeutic Targets in Aggressive Thyroid Cancers. Curr Cancer Drug Targets. 2019;19:561–70. https://doi.org/10.2174/1568009618666180628154727.
Vosgha H, Ariana A, Smith RA, Lam AK. miR-205 targets angiogenesis and EMT concurrently in anaplastic thyroid carcinoma. Endocr Relat Cancer. 2018;25:323–37. https://doi.org/10.1530/ERC-17-0497.
Maroof H, Islam F, Dong L, Ajjikuttira P, Gopalan V, McMillan NAJ, et al. Liposomal Delivery of miR-34b-5p Induced Cancer Cell Death in Thyroid Carcinoma. Cells. 2018;7:265. https://doi.org/10.3390/cells7120265.
Maroof H, Islam F, Ariana A, Gopalan V, Lam AK. The roles of microRNA-34b-5p in angiogenesis of thyroid carcinoma. Endocrine. 2017;58:153–66. https://doi.org/10.1007/s12020-017-1393-3.
Salajegheh A, Vosgha H, Rahman MA, Amin M, Smith RA, Lam AK. Interactive role of miR-126 on VEGF-A and progression of papillary and undifferentiated thyroid carcinoma. Hum Pathol. 2016;51:75–85. https://doi.org/10.1016/j.humpath.2015.12.018.
Yau T, Lo CY, Epstein RJ, Lam AK, Wan KY, Lang BH. Treatment outcomes in anaplastic thyroid carcinoma: survival improvement in young patients with localised disease treated by combination of surgery and radiotherapy. Ann Surg Oncol. 2008;15:2500–5. https://doi.org/10.1245/s10434-008-0005-0.
Pierie JP, Muzikansky A, Gaz RD, Faquin WC, Ott MJ. The effect of surgery and radiotherapy on outcome of anaplastic thyroid carcinoma. Ann Surg Oncol. 2002;9:57–64. https://doi.org/10.1245/aso.2002.9.1.57.
Chang HS, Nam KH, Chung WY, Park CS. Anaplastic thyroid carcinoma: a therapeutic dilemma. Yonsei Med J. 2005;46:759–64. https://doi.org/10.3349/ymj.2005.46.6.759.
Smallridge RC, Ain KB, Asa SL, Bible KC, Brierley JD, Burman KD, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–39. https://doi.org/10.1089/thy.2012.0302.
Haigh PI, Ituarte PH, Wu HS, Treseler PA, Posner MD, Quivey JM, et al. Completely resected anaplastic thyroid carcinoma combined with adjuvant chemotherapy and irradiation is associated with prolonged survival. Cancer. 2001;91:2335–42.
Ain KB, Egorin MJ, DeSimone PA. Treatment of anaplastic thyroid carcinoma with paclitaxel: phase 2 trial using ninety-six-hour infusion. Collaborative Anaplastic Thyroid Cancer Health Intervention Trials (CATCHIT) Group. Thyroid. 2000;10:587–94. https://doi.org/10.1089/thy.2000.10.587.
Higashiyama T, Ito Y, Hirokawa M, Fukushima M, Uruno T, Miya A, et al. Induction chemotherapy with weekly paclitaxel administration for anaplastic thyroid carcinoma. Thyroid. 2010;20:7–14. https://doi.org/10.1089/thy.2009.0115.
Tacara O, Sriamornsak P, Dassa CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–70. https://doi.org/10.1111/j.2042-7158.2012.01567.x.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. https://doi.org/10.1016/j.ejphar.2014.07.025.
Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R. A randomised trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer. 1985;56:2155–60. https://doi.org/10.1002/1097-0142(19851101)56:9<2155::aid-cncr2820560903>3.0.co;2-e.
Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83:288–91. https://doi.org/10.1093/jnci/83.4.288.
Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–34. https://doi.org/10.1126/science.2683079.
Park JE, Woo SR, Kang CM, Juhn KM, Ju YJ, Shin HJ, et al. Paclitaxel stimulates chromosomal fusion and instability in cells with dysfunctional telomeres: implication in multinucleation and chemosensitisation. Biochem Biophys Res Commun. 2011;404:615–21. https://doi.org/10.1016/j.bbrc.2010.12.018.
Onoda N, Sugino K, Higashiyama T, Kammori M, Toda K, Ito K, et al. The Safety and Efficacy of Weekly Paclitaxel Administration for Anaplastic Thyroid Cancer Patients: A Nationwide Prospective Study. Thyroid. 2016;26:1293–9. https://doi.org/10.1089/thy.2016.0072.
Troch M, Koperek O, Scheuba C, Dieckmann K, Hoffmann M, Niederle B, et al. High efficacy of concomitant treatment of undifferentiated (anaplastic) thyroid cancer with radiation and docetaxel. J Clin Endocrinol Metab. 2010;95:E54–7. https://doi.org/10.1210/jc.2009-2827.
Pennell NA, Daniels GH, Haddad RI, Ross DS, Evans T, Wirth LJ, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid. 2008;18:317–23. https://doi.org/10.1089/thy.2007.0120.
Ha HT, Lee JS, Urba S, Koenig RJ, Sisson J, Giordano T, et al. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010;20:975–80. https://doi.org/10.1089/thy.2010.0057.
Bible KC, Suman VJ, Menefee ME, Smallridge RC, Molina JR, Maples WJ, et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97:3179–84. https://doi.org/10.1210/jc.2012-1520.
Cohen EE, Tortorici M, Kim S, Ingrosso A, Pithavala YK, Bycott P. A Phase II trial of axitinib in patients with various histologic subtypes of advanced thyroid cancer: long-term outcomes and pharmacokinetic/pharmacodynamic analyses. Cancer Chemother Pharmacol. 2014;74:1261–70. https://doi.org/10.1007/s00280-014-2604-8.
Ravaud A, de la Fouchardière C, Caron P, Doussau A, Do Cao C, Asselineau J, et al. A multicenter phase II study of sunitinib in patients with locally advanced or metastatic differentiated, anaplastic or medullary thyroid carcinomas: mature data from the THYSU study. Eur J Cancer. 2017;76:110–7. https://doi.org/10.1016/j.ejca.2017.01.029.
Savvides P, Nagaiah G, Lavertu P, Fu P, Wright JJ, Chapman R, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–4. https://doi.org/10.1089/thy.2012.0103.
Ito Y, Onoda N, Ito KI, Sugitani I, Takahashi S, Yamaguchi I, et al. Sorafenib in Japanese Patients with Locally Advanced or Metastatic Medullary Thyroid Carcinoma and Anaplastic Thyroid Carcinoma. Thyroid. 2017;27:1142–8. https://doi.org/10.1089/thy.2016.0621.
Takahashi S, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, et al. A Phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019;15:717–26. https://doi.org/10.2217/fon-2018-0557.
Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N Engl J Med. 2015;373:726–36. https://doi.org/10.1056/NEJMoa1502309.
Schneider TC, de Wit D, Links TP, van Erp NP, van der Hoeven JJ, Gelderblom H, et al. Everolimus in patients with advanced follicular-derived thyroid cancer: Results of a phase II clinical trial. J Clin Endocrinol Metab. 2017;102:698–707. https://doi.org/10.1210/jc.2016-2525.
Harris EJ, Hanna GJ, Chau N, Rabinowits G, Haddad R, Margalit DN, et al. Everolimus in Anaplastic Thyroid Cancer: A Case Series. Front Oncol. 2019;9:106. https://doi.org/10.3389/fonc.2019.00106.
Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–54.
Nobuhara Y, Onoda N, Yamashita Y, Yamasaki M, Ogisawa K, Takashima T, et al. Efficacy of epidermal growth factor receptor-targeted molecular therapy in anaplastic thyroid cancer cell lines. Br J Cancer. 2005;92:1110–6. https://doi.org/10.1038/sj.bjc.6602461.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. https://doi.org/10.1056/NEJM200104053441401.
Krystal GW, Honsawek S, Litz J, Buchdunger E. The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Cancer Res. 2000;6:3319–26.
Podtcheko A, Ohtsuru A, Tsuda S, Namba H, Saenko V, Nakashima M, et al. The selective tyrosine kinase inhibitor, STI571, inhibits growth of anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 2003;88:1889–96. https://doi.org/10.1210/jc.2002-021230.
Dziba JM, Ain KB. Imatinib mesylate (gleevec; STI571) monotherapy is ineffective in suppressing human anaplastic thyroid carcinoma cell growth in vitro. J Clin Endocrinol Metab. 2004;89:2127–35. https://doi.org/10.1210/jc.2003-031734.
Heldin NE, Gustavsson B, Claesson-Welsh L, Hammacher A, Mark J, Heldin CH, et al. Aberrant expression of receptors for platelet-derived growth factor in an anaplastic thyroid carcinoma cell line. Proc Natl Acad Sci U S A. 1988;85:9302–6. https://doi.org/10.1073/pnas.85.23.9302.
Sloan B, Scheinfeld NS. Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr Opin Investig Drugs. 2008;9:1324–35.
Sonpavde G, Hutson TE. Pazopanib: A novel multitargeted tyrosine kinase inhibitor. Curr Oncol Rep. 2007;9:115–9. https://doi.org/10.1007/s11912-007-0007-2.
Bhargava P, Robinson MO. Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep. 2011;13:103–11. https://doi.org/10.1007/s11912-011-0154-3.
Potapova O, Laird AD, Nannini MA, Barone A, Li G, Moss KG, et al. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther. 2006;5:1280–9. https://doi.org/10.1158/1535-7163.MCT-03-0156.
Quek R, George S. Gastrointestinal stromal tumor: a clinical overview. Hematol Oncol Clin North Am. 2009;23:69–78. https://doi.org/10.1016/j.hoc.2008.11.006.
Di Desidero T, Fioravanti A, Orlandi P, Canu B, Giannini R, Borrelli N, et al. Antiproliferative and proapoptotic activity of sunitinib on endothelial and anaplastic thyroid cancer cells via inhibition of Akt and ERK1/2 phosphorylation and by down-regulation of cyclin-D1. J Clin Endocrinol Metab. 2013;98:E1465–73. https://doi.org/10.1210/jc.2013-1364.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. https://doi.org/10.1158/0008-5472.CAN-04-1443.
Kim S, Yazici YD, Calzada G, Wang ZY, Younes MN, Jasser SA, et al. Sorafenib inhibits the angiogenesis and growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Mol Cancer Ther. 2007;6:1785–92. https://doi.org/10.1158/1535-7163.MCT-06-0595.
Ishihara S, Onoda N, Noda S, Asano Y, Tauchi Y, Morisaki T, et al. Sorafenib inhibits vascular endothelial cell proliferation stimulated by anaplastic thyroid cancer cells regardless of BRAF mutation status. Int J Oncol. 2019;55:1069–76. https://doi.org/10.3892/ijo.2019.4881.
Lorusso L, Newbold K. Lenvatinib: a new option for the treatment of advanced iodine refractory differentiated thyroid cancer? Future Oncol. 2015;11:1719–27. https://doi.org/10.2217/fon.15.68.
Ferrari SM, Bocci G, Di Desidero T, Elia G, Ruffilli I, Ragusa F, et al. Lenvatinib exhibits antineoplastic activity in anaplastic thyroid cancer in vitro and in vivo. Oncol Rep. 2018;39:2225–34. https://doi.org/10.3892/or.2018.6306.
Kim G, McKee AE, Ning YM, Hazarika M, Theoret M, Johnson JR, et al. FDA approval summary: vemurafenib for treatment of unresectable or metastatic melanoma with the BRAFV600E mutation. Clin Cancer Res. 2014;20:4994–5000. https://doi.org/10.1158/1078-0432.CCR-14-0776.
Liu W, Kelly JW, Trivett M, Murray WK, Dowling JP, Wolfe R, et al. Distinct clinical and pathological features are associated with the BRAF(T1799A(V600E)) mutation in primary melanoma. J Invest Dermatol. 2007;127:900–5. https://doi.org/10.1038/sj.jid.5700632.
Zhang L, Gaskins K, Yu Z, Xiong Y, Merino MJ, Kebebew E. An in vivo mouse model of metastatic human thyroid cancer. Thyroid. 2014;24:695–704. https://doi.org/10.1089/thy.2013.0149.
Kurata K, Onoda N, Noda S, Kashiwagi S, Asano Y, Hirakawa K, et al. Growth arrest by activated BRAF and MEK inhibition in human anaplastic thyroid cancer cells. Int J Oncol. 2016;49:2303–8. https://doi.org/10.3892/ijo.2016.3723.
• Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Trametinib treatment in patients with locally advanced or metastatic BRAF V600-Mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36:7–13. https://doi.org/10.1200/JCO.2017.73.6785. The FDA approved the combination therapy of dabrafenib and trametinib for treatment in patients with anaplastic thyroid carcinoma having BRAF V600E mutations considering this study.
Alqurashi N, Gopalan V, Smith RA, Lam AK. Clinical impacts of mammalian target of rapamycin expression in human colorectal cancers. Hum Pathol. 2013;44:2089–96. https://doi.org/10.1016/j.humpath.2013.03.014.
Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886–918. https://doi.org/10.3390/ijms13021886.
Papewalis C, Wuttke M, Schinner S, Willenberg HS, Baran AM, Scherbaum WA, et al. Role of the novel mTOR inhibitor RAD001 (everolimus) in anaplastic thyroid cancer. Horm Metab Res. 2009;41:752–6. https://doi.org/10.1055/s-0029-1224116.
Godbert Y, Henriques de Figueiredo B, Bonichon F, Chibon F, Hostein I, Pérot G, et al. Remarkable response to crizotinib in woman with anaplastic lymphoma kinase-rearranged anaplastic thyroid carcinoma. J Clin Oncol. 2015;33:e84–7. https://doi.org/10.1200/JCO.2013.49.6596.
Leroy L, Bonhomme B, Le Moulec S, Soubeyran I, Italiano A, Godbert Y. Remarkable Response to Ceritinib and Brigatinib in an Anaplastic Lymphoma Kinase-Rearranged Anaplastic Thyroid Carcinoma Previously Treated with Crizotinib. Thyroid. 2020;30:343–4. https://doi.org/10.1089/thy.2019.0202.
Dias-Santagata D, Lennerz JK, Sadow PM, Frazier RP, Raju SG, Henry D, et al. Response to RET-Specific Therapy in RET Fusion-Positive Anaplastic Thyroid Carcinoma. Thyroid. 2020;30:1384–9. https://doi.org/10.1089/thy.2019.0477.
Goldstein DA, Chen Q, Ayer T, Chan KKW, Virik K, Hammerman A, et al. Bevacizumab for Metastatic Colorectal Cancer: A Global Cost-Effectiveness Analysis. Oncologist. 2017;22:694–9. https://doi.org/10.1634/theoncologist.2016-0455.
Rossi L, Verrico M, Zaccarelli E, Papa A, Colonna M, Strudel M, et al. Bevacizumab in ovarian cancer: A critical review of phase III studies. Oncotarget. 2017;8:12389–405. https://doi.org/10.18632/oncotarget.13310.
Wakelee HA, Dahlberg SE, Keller SM, Tester WJ, Gandara DR, Graziano SL, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2017;18:1610–23. https://doi.org/10.1016/S1470-2045(17)30691-5.
Garg M, Okamoto R, Nagata Y, Kanojia D, Venkatesan S, Anand MT, et al. Establishment and characterisation of novel human primary and metastatic anaplastic thyroid cancer cell lines and their genomic evolution over a year as a primagraft. J Clin Endocrinol Metab. 2015;100:725–35. https://doi.org/10.1210/jc.2014-2359.
Gomez-Rivera F, Santillan-Gomez AA, Younes MN, Kim S, Fooshee D, Zhao M, et al. The tyrosine kinase inhibitor, AZD2171, inhibits vascular endothelial growth factor receptor signaling and growth of anaplastic thyroid cancer in an orthotopic nude mouse model. Clin Cancer Res. 2007;13:4519–27. https://doi.org/10.1158/1078-0432.CCR-06-2636.
Mooney CJ, Nagaiah G, Fu P, Wasman JK, Cooney MM, Savvides PS, et al. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline serum-soluble intracellular adhesion molecule-1 with outcome. Thyroid. 2009;19:233–40. https://doi.org/10.1089/thy.2008.0321.
Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5:419–29. https://doi.org/10.1016/S1470-2045(04)01509-8.
Hayashi N, Nakamori S, Hiraoka N, Tsujie M, Xundi X, Takano T, et al. Antitumor effects of peroxisome proliferator activate receptor gamma ligands on anaplastic thyroid carcinoma. Int J Oncol. 2004;24:89–95.
Smallridge RC, Copland JA, Brose MS, Wadsworth JT, Houvras Y, Menefee ME, et al. Efatutazone, an oral PPAR-γ agonist, in combination with paclitaxel in anaplastic thyroid cancer: results of a multicenter phase 1 trial. J Clin Endocrinol Metab. 2013;98:2392–400. https://doi.org/10.1210/jc.2013-1106.
Ng HY, Li J, Tao L, Lam AK, Chan KW, Ko JMY, et al. Chemotherapeutic Treatments Increase PD-L1 expression in esophageal squamous cell carcinoma through EGFR/ERK Activation. Transl Oncol. 2018;11:1323–33. https://doi.org/10.1016/j.tranon.2018.08.005.
Mei Z, Huang J, Qiao B, Lam AK. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int J Oral Sci. 2020;12:16. https://doi.org/10.1038/s41368-020-0084-8.
Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–41. https://doi.org/10.1016/S1470-2045(17)30607-1.
Kasem K, Lam AK. Immunohistochemistry for protein detection in esophageal squamous cell carcinoma. Methods Mol Biol. 2020;2129:279–94. https://doi.org/10.1007/978-1-0716-0377-2_21.
Ahn S, Kim TH, Kim SW, Ki CS, Jang HW, Kim JS, et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer. 2017;24:97–106. https://doi.org/10.1530/ERC-16-0421.
Cantara S, Bertelli E, Occhini R, Regoli M, Brilli L, Pacini F, et al. Blockade of the programmed death ligand 1 (PD-L1) as potential therapy for anaplastic thyroid cancer. Endocrine. 2019;64:122–9. https://doi.org/10.1007/s12020-019-01865-5.
Iyer PC, Dadu R, Gule-Monroe M, Busaidy NL, Ferrarotto R, Habra MA, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer. 2018;6:68. https://doi.org/10.1186/s40425-018-0378-y.
• Wang JR, Zafereo ME, Dadu R, Ferrarotto R, Busaidy NL, Lu C, et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAF(V600E)-Mutated Anaplastic Thyroid Carcinoma. Thyroid. 2019;29:1036–43. https://doi.org/10.1089/thy.2019.0133. This study indicated the immunotherapy with the combination of dabrafenib and trametinib could be more effective in patients with anaplastic thyroid carcinoma having BRAF V600E mutations.
Abe I, Karasaki S, Matsuda Y, Sakamoto S, Nakashima T, Yamamoto H, et al. Complete remission of anaplastic thyroid carcinoma after concomitant treatment with docetaxel and radiotherapy. Case Rep Endocrinol. 2015;2015:726085–4. https://doi.org/10.1155/2015/726085.
Shinohara S, Kikuchi M, Naito Y, Fujiwara K, Hori S, Tona Y, et al. Successful treatment of locally advanced anaplastic thyroid carcinoma by chemotherapy and hyperfractionated radiotherapy. Auris Nasus Larynx. 2009;36:729–32. https://doi.org/10.1016/j.anl.2009.02.001.
Noguchi H, Yamashita H, Murakami T, Hirai K, Noguchi Y, Maruta J, et al. Successful treatment of anaplastic thyroid carcinoma with a combination of oral valproic acid, chemotherapy, radiation and surgery. Endocr J. 2009;56:245–9. https://doi.org/10.1507/endocrj.k08e-016.
Zanirato Rambaldi G, Monari F, Fiorentino M, Cammelli S, Repaci A, Cremonini N, et al. Complete pathological response after chemo-radiation in anaplastic thyroid cancer: A report of two cases. Acta Oncol. 2016;55:530–2. https://doi.org/10.3109/0284186X.2015.1102966.
Pichardo-Lowden A, Durvesh S, Douglas S, Todd W, Bruno M, Goldenberg D. Anaplastic thyroid carcinoma in a young woman: a rare case of survival. Thyroid. 2009;19:775–9. https://doi.org/10.1089/thy.2009.0025.
Xing JC, Bishop JA, Mathioudakis N, Agrawal N, Tufano RP. A large nonmetastatic anaplastic thyroid cancer with complete thyroidal confinement. Case Rep Med. 2011;2011:583978–4. https://doi.org/10.1155/2011/583978.
Koussis H, Giorgi CA, Di Liso E, Carlucci MC, Fassina A, Marioni G, et al. Complete response to weekly carboplatin-docetaxel chemotherapy in a 91-year-old woman with anaplastic thyroid cancer. Am J Otolaryngol. 2015;36:268–72. https://doi.org/10.1016/j.amjoto.2014.03.006.
Kurukahvecioglu O, Ege B, Poyraz A, Tezel E, Taneri F. Anaplastic thyroid carcinoma with long term survival after combined treatment: case report. Endocr Regul. 2007;41:41–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors has any potential conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ichiro Abe and Alfred King-yin Lam contributes equally as co-principal authors
This article is part of the Topical Collection on Head and Neck Cancers
Rights and permissions
About this article
Cite this article
Abe, I., Lam, A.Ky. Anaplastic Thyroid Carcinoma: Current Issues in Genomics and Therapeutics. Curr Oncol Rep 23, 31 (2021). https://doi.org/10.1007/s11912-021-01019-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01019-9