Abstract
Purpose of Review
The purpose of the study is to summarize the current conundrums in the management of marginal zone lymphomas (MZL).
Recent Findings
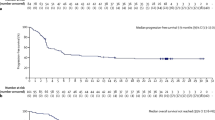
In 2017, the US Food and Drug Administration (FDA) approved ibrutinib, a first in class Bruton Tyrosine Kinase inhibitor, for the treatment of relapsed/refractory MZL based on pivotal open-label phase II trial demonstrating an overall response rates of 48%. Clinical trials design utilizing chemotherapy-free regimens for relapsed/refractory disease are gaining popularity. Recent studies have identified multiple genetic biomarkers that helped characterize and prognosticate different subtypes of MZL.
Summary
MZLs are heterogeneous, mostly indolent, malignancies derived from B lymphocytes. Three disease subtypes are recognized, extranodal, nodal, and splenic. The disease characteristics, clinical picture, and treatment algorithms vary considerably based on subtype and site of involvement. Recent discoveries have enhanced our knowledge of the pathogenesis of MZLs leading to development of more accurate prognostic models as well as novel targeted systemic therapies.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
A clinical evaluation of the international lymphoma study group classification of non-Hodgkin’s lymphoma. Blood 1997; 89(11):3909–18.
Liu L, Wang H, Chen Y, Rustveld L, Liu G, Du XL. Splenic marginal zone lymphoma: a population-based study on the 2001-2008 incidence and survival in the United States. Leuk Lymphoma. 2013;54(7):1380–6. https://doi.org/10.3109/10428194.2012.743655.
Berger F, Felman P, Thieblemont C, Pradier T, Baseggio L, Bryon PA, et al. Non-MALT marginal zone B-cell lymphomas: a description of clinical presentation and outcome in 124 patients. Blood. 2000;95(6):1950–6.
Chacon JI, Mollejo M, Munoz E, Algara P, Mateo M, Lopez L, et al. Splenic marginal zone lymphoma: clinical characteristics and prognostic factors in a series of 60 patients. Blood. 2002;100(5):1648–54.
• Arcaini L, Lazzarino M, Colombo N, Burcheri S, Boveri E, Paulli M, et al. Splenic marginal zone lymphoma: a prognostic model for clinical use. Blood. 2006;107(12):4643–9. https://doi.org/10.1182/blood-2005-11-4659. One of the earliest models for prognostication of SMZL by The Integruppo Italiano Linfomi (IIL) based on a large case series of 309 patients.
Salido M, Baro C, Oscier D, Stamatopoulos K, Dierlamm J, Matutes E, et al. Cytogenetic aberrations and their prognostic value in a series of 330 splenic marginal zone B-cell lymphomas: a multicenter study of the splenic B-cell lymphoma group. Blood. 2010;116(9):1479–88. https://doi.org/10.1182/blood-2010-02-267476.
Swerdlow SH, Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Vardiman, J.W. WHO classification of tumours of haematopoietic and lymphoid tissues, fourth edition. IARC. 2008.
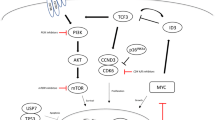
• Parry M, Rose-Zerilli MJJ, Ljungström V, Gibson J, Wang J, Walewska R, et al. Genetics and prognostication in splenic marginal zone lymphoma: revelations from deep sequencing. Clin Cancer Res. 2015;21(18):4174. Recent study describing genetic changes that help prognosticate SMZL–83.
• Kiel MJ, Velusamy T, Betz BL, Zhao L, Weigelin HG, Chiang MY, et al. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J Exp Med. 2012;209(9):1553–65. https://doi.org/10.1084/jem.20120910. Recent study describing genetic changes that help prognosticate SMZL.
• Piva R, Deaglio S, Fama R, Buonincontri R, Scarfo I, Bruscaggin A, et al. The Kruppel-like factor 2 transcription factor gene is recurrently mutated in splenic marginal zone lymphoma. Leukemia. 2015;29(2):503–7. https://doi.org/10.1038/leu.2014.294. Recent study describing genetic changes that help prognosticate SMZL.
• Clipson A, Wang M, de Leval L, Ashton-Key M, Wotherspoon A, Vassiliou G, et al. KLF2 mutation is the most frequent somatic change in splenic marginal zone lymphoma and identifies a subset with distinct genotype. Leukemia. 2015;29(5):1177–85. https://doi.org/10.1038/leu.2014.330. Recent study describing genetic changes that help prognosticate SMZL.
Rossi D, Trifonov V, Fangazio M, Bruscaggin A, Rasi S, Spina V, et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J Exp Med. 2012;209(9):1537–51. https://doi.org/10.1084/jem.20120904.
Dierlamm J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld DK, et al. The apoptosis inhibitor gene <em>API2</em> and a novel 18q gene,<em>MLT,</em> are recurrently rearranged in the t(11;18)(q21;q21) associated with mucosa-associated lymphoid tissue lymphomas. Blood. 1999;93(11):3601–9.
Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19(4):652–8. https://doi.org/10.1038/sj.leu.2403644.
• Spina V, Khiabanian H, Messina M, Monti S, Cascione L, Bruscaggin A, et al. The genetics of nodal marginal zone lymphoma. Blood. 2016;128(10):1362–73. https://doi.org/10.1182/blood-2016-02-696757. Recent report of genetic changes unique to NMZL.
Kiel MJ, Velusamy T, Betz BL, Zhao L, Weigelin HG, Chiang MY, et al. Whole-genome sequencing identifies recurrent somatic <em>NOTCH2</em> mutations in splenic marginal zone lymphoma. J Exp Med. 2012;209(9):1553–65.
Martinez N, Almaraz C, Vaque JP, Varela I, Derdak S, Beltran S, et al. Whole-exome sequencing in splenic marginal zone lymphoma reveals mutations in genes involved in marginal zone differentiation. Leukemia. 2014;28(6):1334–40. https://doi.org/10.1038/leu.2013.365.
Parry M, Rose-Zerilli MJ, Gibson J, Ennis S, Walewska R, Forster J, et al. Whole exome sequencing identifies novel recurrently mutated genes in patients with splenic marginal zone lymphoma. PLoS One. 2013;8(12):e83244. https://doi.org/10.1371/journal.pone.0083244.
• Rossi D, Deaglio S, Dominguez-Sola D, Rasi S, Vaisitti T, Agostinelli C, et al. Alteration of BIRC3 and multiple other NF-kappaB pathway genes in splenic marginal zone lymphoma. Blood. 2011;118(18):4930–4. https://doi.org/10.1182/blood-2011-06-359166. Alteration of NF-kB pathway in SMZL.
Martinez-Lopez A, Curiel-Olmo S, Mollejo M, Cereceda L, Martinez N, Montes-Moreno S, et al. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. Am J Surg Pathol. 2015;39(5):644–51. https://doi.org/10.1097/pas.0000000000000411.
Peveling-Oberhag J, Wolters F, Doring C, Walter D, Sellmann L, Scholtysik R, et al. Whole exome sequencing of microdissected splenic marginal zone lymphoma: a study to discover novel tumor-specific mutations. BMC Cancer. 2015;15:773. https://doi.org/10.1186/s12885-015-1766-z.
Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, et al. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96(1):35–45.
• Streubel B, Lamprecht A, Dierlamm J, Cerroni L, Stolte M, Ott G, et al. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood. 2003;101(6):2335–9. https://doi.org/10.1182/blood-2002-09-2963.
Algara P, Mateo MS, Sanchez-Beato M, Mollejo M, Navas IC, Romero L, et al. Analysis of the IgV(H) somatic mutations in splenic marginal zone lymphoma defines a group of unmutated cases with frequent 7q deletion and adverse clinical course. Blood. 2002;99(4):1299–304.
Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127(17):2072–81.
•• Hermine O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347(2):89–94. https://doi.org/10.1056/NEJMoa013376. Initial report documeting regression of SMZL after HCV therapy.
Troussard X, Valensi F, Duchayne E, Garand R, Felman P, Tulliez M, et al. Splenic lymphoma with villous lymphocytes: clinical presentation, biology and prognostic factors in a series of 100 patients. Groupe Francais d'Hematologie Cellulaire (GFHC). Br J Haematol. 1996;93(3):731–6.
Camacho FI, Mollejo M, Mateo MS, Algara P, Navas C, Hernandez JM, et al. Progression to large B-cell lymphoma in splenic marginal zone lymphoma: a description of a series of 12 cases. Am J Surg Pathol. 2001;25(10):1268–76.
•• Montalban C, Abraira V, Arcaini L, Domingo-Domenech E, Guisado-Vasco P, Iannitto E, et al. Risk stratification for splenic marginal zone lymphoma based on haemoglobin concentration, platelet count, high lactate dehydrogenase level and extrahilar lymphadenopathy: development and validation on 593 cases. Br J Haematol. 2012;159(2):164–71. https://doi.org/10.1111/bjh.12011. HPLL prognostic scheme report of the international collaboration of SMZLSG of 593 patients. The largest case series published to date.
Starr AG, Caimi PF, Fu P, Massoud MR, Meyerson H, Hsi ED, et al. Splenic marginal zone lymphoma: excellent outcomes in 64 patients treated in the rituximab era. Hematology. 2017;22(7):405–11. https://doi.org/10.1080/10245332.2017.1279842.
Heilgeist A, McClanahan F, Ho AD, Witzens-Harig M. Prognostic value of the follicular lymphoma international prognostic index score in marginal zone lymphoma: an analysis of clinical presentation and outcome in 144 patients. Cancer. 2013;119(1):99–106. https://doi.org/10.1002/cncr.27704.
•• Rosado MF, Byrne GE Jr, Ding F, Fields KA, Ruiz P, Dubovy SR, et al. Ocular adnexal lymphoma: a clinicopathologic study of a large cohort of patients with no evidence for an association with Chlamydia psittaci. Blood. 2006;107(2):467–72. https://doi.org/10.1182/blood-2005-06-2332. One of the largest cohorts of ocular adnexal MZL showing associatin with C. psittaci
Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: factors relevant to prognosis. Gastroenterology. 1992;102(5):1628–38.
Akamatsu T, Mochizuki T, Okiyama Y, Matsumoto A, Miyabayashi H, Ota H. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter. 2006;11(2):86–95. https://doi.org/10.1111/j.1523-5378.2006.00382.x.
Cerroni L, Zochling N, Putz B, Kerl H. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J Cutan Pathol. 1997;24(8):457–61.
Chanudet E, Zhou Y, Bacon CM, Wotherspoon AC, Muller-Hermelink HK, Adam P, et al. Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J Pathol. 2006;209(3):344–51. https://doi.org/10.1002/path.1984.
Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98(1):51–60. https://doi.org/10.1093/jnci/djj004.
Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113(3):321–33. https://doi.org/10.1172/JCI20925.
Yamazaki S, Yamakawa A, Ito Y, Ohtani M, Higashi H, Hatakeyama M, et al. The CagA protein of Helicobacter pylori is translocated into epithelial cells and binds to SHP-2 in human gastric mucosa. J Infect Dis. 2003;187(2):334–7. https://doi.org/10.1086/367807.
Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8(2):105–10. https://doi.org/10.1016/j.cgh.2009.07.017.
• Nakamura S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61(4):507–13. https://doi.org/10.1136/gutjnl-2011-300495. Large cohort from Japan showing excellent outcomes of gastric MALT after H. pylori eradication.
Murga Penas EM, Hinz K, Roser K, Copie-Bergman C, Wlodarska I, Marynen P, et al. Translocations t(11;18)(q21;q21) and t(14;18)(q32;q21) are the main chromosomal abnormalities involving MLT/MALT1 in MALT lymphomas. Leukemia. 2003;17(11):2225–9. https://doi.org/10.1038/sj.leu.2403122.
Ho L, Davis RE, Conne B, Chappuis R, Berczy M, Mhawech P, et al. MALT1 and the API2-MALT1 fusion act between CD40 and IKK and confer NF-kappa B-dependent proliferative advantage and resistance against FAS-induced cell death in B cells. Blood. 2005;105(7):2891–9. https://doi.org/10.1182/blood-2004-06-2297.
Rosebeck S, Madden L, Jin X, Gu S, Apel IJ, Appert A, et al. Cleavage of NIK by the API2-MALT1 fusion oncoprotein leads to noncanonical NF-kappaB activation. Science. 2011;331(6016):468–72. https://doi.org/10.1126/science.1198946.
• Ye H, Liu H, Raderer M, Chott A, Ruskone-Fourmestraux A, Wotherspoon A, et al. High incidence of t(11;18)(q21;q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood. 2003;101(7):2547–50. https://doi.org/10.1182/blood-2002-10-3167. Initial report of t(11;18) associating H. pylori - negative gastric MALT.
•• Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. 2001;357(9249):39–40. https://doi.org/10.1016/s0140-6736(00)03571-6. Initial report of t(11;18) as a marker of resistance of gastric MALT to H. pylori eradication.
• Sagaert X, de Paepe P, Libbrecht L, Vanhentenrijk V, Verhoef G, Thomas J, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(16):2490–7. https://doi.org/10.1200/jco.2006.05.6150. Initial report of Forkhead box protein P1 as marker of poor outcomes in EMZL.
• Teckie S, Qi S, Lovie S, Navarrett S, Hsu M, Noy A, et al. Long-term outcomes and patterns of relapse of early-stage extranodal marginal zone lymphoma treated with radiation therapy with curative intent. Int J Radiat Oncol Biol Phys. 2015;92(1):130–7. https://doi.org/10.1016/j.ijrobp.2015.01.040. Long term follow up report of excellent long term outcomes after radiation in early stage EMZL.
• Thieblemont C, Cascione L, Conconi A, Kiesewetter B, Raderer M, Gaidano G, et al. A MALT lymphoma prognostic index. Blood. 2017;130(12):1409. MALT lymphoma prognostic index.
Starr AG, Caimi PF, Fu P, Massoud MR, Meyerson H, Hsi ED, et al. Dual institution experience of extranodal marginal zone lymphoma reveals excellent long-term outcomes. Br J Haematol. 2016;173(3):404–12. https://doi.org/10.1111/bjh.13975.
Camacho FI, Algara P, Mollejo M, Garcia JF, Montalban C, Martinez N, et al. Nodal marginal zone lymphoma: a heterogeneous tumor: a comprehensive analysis of a series of 27 cases. Am J Surg Pathol. 2003;27(6):762–71.
Starr AG, Caimi PF, Fu P, Massoud MR, Meyerson H, Hsi ED, et al. Dual institution experience of nodal marginal zone lymphoma reveals excellent long-term outcomes in the rituximab era. Br J Haematol. 2016;175(2):275–80. https://doi.org/10.1111/bjh.14228.
Traverse-Glehen A, Davi F, Ben Simon E, Callet-Bauchu E, Felman P, Baseggio L, et al. Analysis of VH genes in marginal zone lymphoma reveals marked heterogeneity between splenic and nodal tumors and suggests the existence of clonal selection. Haematologica. 2005;90(4):470–8.
Marasca R, Vaccari P, Luppi M, Zucchini P, Castelli I, Barozzi P, et al. Immunoglobulin gene mutations and frequent use of VH1-69 and VH4-34 segments in hepatitis C virus-positive and hepatitis C virus-negative nodal marginal zone B-cell lymphoma. Am J Pathol. 2001;159(1):253–61. https://doi.org/10.1016/s0002-9440(10)61691-4.
Hart GT, Wang X, Hogquist KA, Jameson SC. Kruppel-like factor 2 (KLF2) regulates B-cell reactivity, subset differentiation, and trafficking molecule expression. Proc Natl Acad Sci U S A. 2011;108(2):716–21. https://doi.org/10.1073/pnas.1013168108.
Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9(11):767–77. https://doi.org/10.1038/nri2656.
Oh SY, Ryoo BY, Kim WS, Kim K, Lee J, Kim HJ, et al. Nodal marginal zone B-cell lymphoma: analysis of 36 cases. Clinical presentation and treatment outcomes of nodal marginal zone B-cell lymphoma. Ann Hematol. 2006;85(11):781–6. https://doi.org/10.1007/s00277-006-0160-y.
Arcaini L, Paulli M, Burcheri S, Rossi A, Spina M, Passamonti F, et al. Primary nodal marginal zone B-cell lymphoma: clinical features and prognostic assessment of a rare disease. Br J Haematol. 2007;136(2):301–4. https://doi.org/10.1111/j.1365-2141.2006.06437.x.
Yahalom J, Illidge T, Specht L, Hoppe RT, Li YX, Tsang R, et al. Modern radiation therapy for extranodal lymphomas: field and dose guidelines from the international lymphoma radiation oncology group. Int J Radiat Oncol Biol Phys. 2015;92(1):11–31. https://doi.org/10.1016/j.ijrobp.2015.01.009.
Ferreri AJ, Ponzoni M, Guidoboni M, Resti AG, Politi LS, Cortelazzo S, et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: a multicenter prospective trial. J Natl Cancer Inst. 2006;98(19):1375–82. https://doi.org/10.1093/jnci/djj373.
•• Ferreri AJ, Govi S, Pasini E, Mappa S, Bertoni F, Zaja F, et al. Chlamydophila psittaci eradication with doxycycline as first-line targeted therapy for ocular adnexae lymphoma: final results of an international phase II trial. J Clin Oncol. 2012;30(24):2988–94. https://doi.org/10.1200/jco.2011.41.4466. International phase II trial documenting remission of ocular adnexae lymphoma after C. psittaci eradication with doxycycline.
•• Wundisch T, Thiede C, Morgner A, Dempfle A, Gunther A, Liu H, et al. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005;23(31):8018–24. https://doi.org/10.1200/jco.2005.02.3903. One of the largest long-term follow up reports from Europe (median follow up 6 years) showing excellent outcomes after H. pylori eradication and documenting t(11;18) as a maker of resistance to eradication.
• Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342(8871):575–7. Initial report of regression of gastric MALT after H. pylori eradication in 6 patients.
Tsang RW, Gospodarowicz MK. Radiation therapy for localized low-grade non-Hodgkin's lymphomas. Hematol Oncol. 2005;23(1):10–7. https://doi.org/10.1002/hon.743.
Mulligan SP, Matutes E, Dearden C, Catovsky D. Splenic lymphoma with villous lymphocytes: natural history and response to therapy in 50 cases. Br J Haematol. 1991;78(2):206–9.
• Lenglet J, Traulle C, Mounier N, Benet C, Munoz-Bongrand N, Amorin S, et al. Long-term follow-up analysis of 100 patients with splenic marginal zone lymphoma treated with splenectomy as first-line treatment. Leuk Lymphoma. 2014;55(8):1854–60. https://doi.org/10.3109/10428194.2013.861067. One of the largest case series and longest follow-up for splenectomy of SMZL.
Olszewski AJ. Survival outcomes with and without splenectomy in splenic marginal zone lymphoma. Am J Hematol. 2012;87(11):E119–E22. https://doi.org/10.1002/ajh.23314.
Tsimberidou AM, Catovsky D, Schlette E, O'Brien S, Wierda WG, Kantarjian H, et al. Outcomes in patients with splenic marginal zone lymphoma and marginal zone lymphoma treated with rituximab with or without chemotherapy or chemotherapy alone. Cancer. 2006;107(1):125–35. https://doi.org/10.1002/cncr.21931.
Kalpadakis C, Pangalis GA, Angelopoulou MK, Sachanas S, Kontopidou FN, Yiakoumis X, et al. Treatment of splenic marginal zone lymphoma with rituximab monotherapy: progress report and comparison with splenectomy. Oncologist. 2013;18(2):190–7. https://doi.org/10.1634/theoncologist.2012-0251.
Bennett M, Sharma K, Yegena S, Gavish I, Dave HP, Schechter GP. Rituximab monotherapy for splenic marginal zone lymphoma. Haematologica. 2005;90(6):856–8.
Else M, Marín-Niebla A, de la Cruz F, Batty P, Ríos E, Dearden CE, et al. Rituximab, used alone or in combination, is superior to other treatment modalities in splenic marginal zone lymphoma. Br J Haematol. 2012;159(3):322–8. https://doi.org/10.1111/bjh.12036.
Kalpadakis C, Pangalis GA, Dimopoulou MN, Vassilakopoulos TP, Kyrtsonis MC, Korkolopoulou P, et al. Rituximab monotherapy is highly effective in splenic marginal zone lymphoma. Hematol Oncol. 2007;25(3):127–31. https://doi.org/10.1002/hon.820.
Arcaini L, Vallisa D, Rattotti S, Ferretti VV, Ferreri AJ, Bernuzzi P, et al. Antiviral treatment in patients with indolent B-cell lymphomas associated with HCV infection: a study of the Fondazione Italiana Linfomi. Ann Oncol. 2014;25(7):1404–10. https://doi.org/10.1093/annonc/mdu166.
Cheson BD, Friedberg JW, Kahl BS, Van der Jagt RH, Tremmel L. Bendamustine produces durable responses with an acceptable safety profile in patients with rituximab-refractory indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2010;10(6):452–7. https://doi.org/10.3816/CLML.2010.n.079.
•• Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–10. https://doi.org/10.1016/S0140-6736(12)61763-2. Landmark randomized phase III trial of the German Study group indolent Lymphomas (StiL) showing superiority of BR over R-CHOP as frontline treatment of indolent lymphoma.
•• Flinn IW, van der Jagt R, Kahl BS, Wood P, Hawkins TE, Macdonald D, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014;123(19):2944–52. https://doi.org/10.1182/blood-2013-11-531327. International phase III trial showing very similar results to the StiL trial (ref. 65 above) based on StiL and BRIGHT data, BR became the preferred upfront agent in indolent lymphomas.
Salar A, Domingo-Domenech E, Panizo C, Nicolás C, Bargay J, Muntañola A, et al. Long-term results of the multicenter phase ii trial with BENDAMUSTINE and rituximab as FIRST line treatment for patients with malt lymphoma (MALT-2008-01). Hematol Oncol. 2017;35:147–8. https://doi.org/10.1002/hon.2437_137.
Kang HJ, Kim WS, Kim SJ, Lee JJ, Yang DH, Kim JS, et al. Phase II trial of rituximab plus CVP combination chemotherapy for advanced stage marginal zone lymphoma as a first-line therapy: consortium for improving survival of lymphoma (CISL) study. Ann Hematol. 2012;91(4):543–51. https://doi.org/10.1007/s00277-011-1337-6.
Brown JR, Friedberg JW, Feng Y, Scofield S, Phillips K, Dal Cin P, et al. A phase 2 study of concurrent fludarabine and rituximab for the treatment of marginal zone lymphomas. Br J Haematol. 2009;145(6):741–8. https://doi.org/10.1111/j.1365-2141.2009.07677.x.
Ferrario A, Pulsoni A, Olivero B, Rossi G, Vitolo U, Tedeschi A, et al. Fludarabine, cyclophosphamide, and rituximab in patients with advanced, untreated, indolent B-cell nonfollicular lymphomas: phase 2 study of the Italian Lymphoma Foundation. Cancer. 2012;118(16):3954–61. https://doi.org/10.1002/cncr.26708.
• Zucca E, Conconi A, Martinelli G, Bouabdallah R, Tucci A, Vitolo U, et al. Final Results of the IELSG-19 Randomized Trial of Mucosa-Associated Lymphoid Tissue Lymphoma: Improved Event-Free and Progression-Free Survival With Rituximab Plus Chlorambucil Versus Either Chlorambucil or Rituximab Monotherapy. J Clin Oncol. 2017;35(17):1905–12. https://doi.org/10.1200/jco.2016.70.6994. Randomized trial showing superiority of chlorambucil in combination with rituximab over either alone in MALT lymphoma failing local or H. pylori eradication therapy.
• Sehn LH, Chua N, Mayer J, Dueck G, Trněný M, Bouabdallah K, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. The Lancet Oncology. 17(8):1081–93. https://doi.org/10.1016/S1470-2045(16)30097-3. Recent trial showing addition of obinutuzumab to bendamustine is superior to bendamustine alone in patients with idolent lymphomas who are rituximab refractory.
•• Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224–32. https://doi.org/10.1182/blood-2016-10-747345. Recent landmark phase II trial showing single-agent ibrutinib induced durable remissions with a favorable benefit-risk profile in patients with previously treated MZL. Trial led to FDA approval of ibrutinib to be the first agent in history specifically approved by the FDA for MZL.
Conconi A, Martinelli G, Lopez-Guillermo A, Zinzani PL, Ferreri AJ, Rigacci L, et al. Clinical activity of bortezomib in relapsed/refractory MALT lymphomas: results of a phase II study of the international extranodal lymphoma study group (IELSG). Ann Oncol. 2011;22(3):689–95. https://doi.org/10.1093/annonc/mdq416.
de Vos S, Goy A, Dakhil SR, Saleh MN, McLaughlin P, Belt R, et al. Multicenter randomized phase II study of weekly or twice-weekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J Clin Oncol. 2009;27(30):5023–30. https://doi.org/10.1200/JCO.2008.17.7980.
• Kiesewetter B, Willenbacher E, Willenbacher W, Egle A, Neumeister P, Voskova D, et al. A phase II study of rituximab plus lenalidomide for mucosa-associated lymphoid tissue lymphoma (MALT lymphoma). Blood. 2016; https://doi.org/10.1182/blood-2016-06-720599. Recent trial confirming activity of lenalidomide with rituximab in MALT lymphoma.
• Andorsky DJ, Yacoub A, Bitran JD, Melear J, Brooks HD, Foon KA, et al. MAGNIFY: phase iiib randomized study of lenalidomide plus rituximab (R<sup>2</sup>) followed by lenalidomide vs. rituximab maintenance in subjects with relapsed/refractory follicular, marginal zone, or mantle cell lymphoma. Blood. 2016;128(22):1798. Recent trial confirming activity of lenalidomide with rituximab in indolent lymphomas.
Gerecitano JF, Roberts AW, Seymour JF, Wierda WG, Kahl BS, Pagel JM, et al. A phase 1 study of venetoclax (ABT-199/GDC-0199) monotherapy in patients with relapsed/refractory non-Hodgkin lymphoma. Blood. 2015;126(23):254.
Dreyling M, Cunningham D, Bouabdallah K, Assouline S, Van den Neste E, Vitolo U, et al. Phase 2A study of copanlisib, a novel PI3K inhibitor, in patients with indolent lymphoma. Blood. 2014;124(21):1701.
Maddocks KJ, Cohen JB, Huang Y, Christian BA, Benson DM, Jones J et al. A phase I study of BKM120 (Buparlisib) and rituximab in patients with relapsed or refractory (R/R) B-cell non-Hodgkin's lymphoma (NHL). Blood 2016;128(22):1776.
Zinzani P, Wagner-Johnston N, Miller C, Ardeshna K, Tertreault S, Assouline S, et al. DYNAMO: a phase 2 study demonstrating the clinical activity of duvelisib in patients with double-refractory indolent non-Hodgkin lymphoma. Hematol Oncol. 2017;35:69–70. https://doi.org/10.1002/hon.2437_57.
Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–18. https://doi.org/10.1056/NEJMoa1314583.
Kiesewetter B, Troch M, Dolak W, Mullauer L, Lukas J, Zielinski CC, et al. A phase II study of lenalidomide in patients with extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT lymphoma). Haematologica. 2013;98(3):353–6. https://doi.org/10.3324/haematol.2012.065995.
Fowler NH, Davis RE, Rawal S, Nastoupil L, Hagemeister FB, McLaughlin P, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15(12):1311–8. https://doi.org/10.1016/s1470-2045(14)70455-3.
Conconi A, Martinelli G, Lopez-Guillermo A, Zinzani PL, Ferreri AJ, Rigacci L, et al. Clinical activity of bortezomib in relapsed/refractory MALT lymphomas: results of a phase II study of the international extranodal lymphoma study group (IELSG). Ann Oncol. 2011;22(3):689–95. https://doi.org/10.1093/annonc/mdq416.
Kirschbaum M, Frankel P, Popplewell L, Zain J, Delioukina M, Pullarkat V, et al. Phase II study of Vorinostat for treatment of relapsed or refractory indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29(9):1198–203. https://doi.org/10.1200/JCO.2010.32.1398.
Herold M, Hoster E, Janssens A, McCarthy H, Tedeschi A, Pocock C, et al. Immunochemotherapy with obinutuzumab or rituximab in a subset of patients in the randomised gallium trial with previously untreated marginal zone lymphoma (MZL). Hematol Oncol. 2017;35:146–7. https://doi.org/10.1002/hon.2437_136.
• Nastoupil L, Lunning MA, Vose JM, Schreeder MT, Siddiqi T, Flowers CR, et al. Chemo-free triplet combination of TGR-1202, ublituximab, and ibrutinib is well tolerated and highly active in patients with advanced CLL and NHL. Hematol Oncol. 2017;35:112–3. https://doi.org/10.1002/hon.2437_101. Very recent chemotherapy-free triplet regimen for indolent lymphoma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sabarish Ayyappan and Basem M. William declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Lymphomas
Rights and permissions
About this article
Cite this article
Ayyappan, S., William, B.M. Marginal Zone Lymphoma: Clinicopathologic Variations and Approaches to Therapy. Curr Oncol Rep 20, 33 (2018). https://doi.org/10.1007/s11912-018-0687-9
Published:
DOI: https://doi.org/10.1007/s11912-018-0687-9