Abstract
Purpose of Review
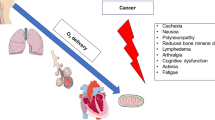
Cancer patients nearly universally experience a decline in quality of life, with fatigue and reduced exercise tolerance as cardinal reflections. A routine exercise program can improve these signs and symptoms as well as overall outcomes. The review provides an updated overview of the field and its translation to clinical practice.
Recent Findings
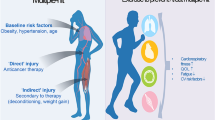
A wealth of clinical studies have documented the safety and benefits of exercise after and during cancer therapy, and pilot and larger-scale studies are currently ongoing to integrate exercise into the treatment program for cancer patients undergoing active therapy (EXACT pilot, OptiTrain, and TITAN study). More recently, efforts have emerged to commence exercise programs before the start of cancer therapy, so-called pre-habilitation. The concept of increasing the cardiovascular reserve beforehand is intuitively attractive. In agreement, preclinical studies support exercise as an effective preventive means before and during cardiotoxic drug exposure. Assuming that a pronounced drop in exercise tolerance will occur during cancer therapy, pre-habilitation can potentially curtail or raise the nadir level of exercise tolerance. Furthermore, such efforts might serve as pre-conditioning efforts in reducing not only the nadir, but even the magnitude of drop in cardiovascular reserve. Initiated beforehand, cancer patients are also more likely to continue these efforts during cancer therapy. Finally, an active exercise routine (≥ 150 min/week moderate intensity or ≥ 75 min/week vigorous intensity or combination) in conjunction with the other six American Heart Association’s cardiovascular health metrics (BMI < 25 kg/m2, blood pressure < 120/80 mmHg, fasting plasma glucose < 100 mg/dL, total cholesterol < 200 mg/dL, 4–5 component healthy diet, no smoking) reduces not only the cardiovascular but also the cancer disease risk.
Summary
Exercise can reduce the risks of developing cancer, the detrimental effects of its treatment on the cardiovascular system, and overall morbidity and mortality. Exercise should become an integral part of the care for every cancer patient.






Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–60.
Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10.
Blanchard CM, Courneya KS, Stein K, American Cancer Society’s SCS-II. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–204.
Buffart LM, Galvão DA, Brug J, Chinapaw MJM, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40(2):327–40.
Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74.
Al-Kindi SG, Oliveira GH. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: the unmet need for onco-cardiology. Mayo Clin Proc. 2016;91(1):81–3.
Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–14.
Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, et al. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127(12):1270–5.
Dong C, Rundek T, Wright CB, Anwar Z, Elkind MSV, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125(24):2975–84.
Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, et al. American Heart Association’s Life’s Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128(9):970–6. e2
Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125(8):987–95.
Florido R, Zhao D, Ndumele CE, Lutsey PL, McEvoy JW, Windham BG, et al. Physical activity, parental history of premature coronary heart disease, and incident atherosclerotic cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) Study. J Am Heart Assoc. 2016;5(9):e003505.
Maessen MF, et al. Lifelong exercise patterns and cardiovascular health. Mayo Clin Proc. 2016;91(6):745–54.
Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176(6):816–25.
Khan NF, Mant D, Carpenter L, Forman D, Rose PW. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer. 2011;105(Suppl 1):S29–37.
Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64.
Wang F, Gulati R, Lennon RJ, Lewis BR, Park J, Sandhu GS, et al. Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Mayo Clin Proc. 2016;91(12):1680–92.
Squires, R.W. (2010) Physical activity and exercise in cardiovascular prevention and rehabilitation. In Evidence based cardiology. (3rd edn) (Yusuf S, Camm AJ, Fallen EL, Gersh B ed), pp. 190–200, Oxford: Wiley-Blackwell.
O’Neill JO, Young JB, Pothier CE, Lauer MS. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation. 2005;111(18):2313–8.
Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell T, Piña IL, et al. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol. 2016;67(7):780–9.
Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–7.
• Klassen O, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol. 2014;53(10):1356–65. Important study in over 200 breast cancer patients with comprehensive cardiopulmonary exercise testing documenting not only a generally much lower than expected level of aerobic capacity (fitness) in breast cancer patients, but also and, in particular, its decline with chemotherapy.
Christiansen JR, Kanellopoulos A, Lund MB, Massey R, Dalen H, Kiserud CE, et al. Impaired exercise capacity and left ventricular function in long-term adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62(8):1437–43.
Armenian SH, Horak D, Scott JM, Mills G, Siyahian A, Berano Teh J, et al. Cardiovascular function in long-term hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2017;23(4):700–5.
Kenjale AA, Hornsby WE, Crowgey T, Thomas S, Herndon JE, Khouri MG, et al. Pre-exercise participation cardiovascular screening in a heterogeneous cohort of adult cancer patients. Oncologist. 2014;19(9):999–1005.
Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–53.
Ross R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653–99.
Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1451–9.
Keteyian SJ, Leifer ES, Houston-Miller N, Kraus WE, Brawner CA, O'Connor CM, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60(19):1899–905.
Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67(1):1–12.
Smith SC Jr, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J Am Coll Cardiol. 2011;58(23):2432–46.
•• Jones LW, et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34(23):2743–9. Cohort study of nearly 3000 breast cancer patients selected from two registry-based, regional cohort studies documenting an inverse relationship between leisure-time recreational physical activity, measured in metabolic equivalent task [MET]-hours per week, and cardiovascular events. Patients meeting the national exercise guidelines for adult patients with cancer (i.e., ≥ 9 MET-h/week) had 23% lower adjusted risk of cardiovascular events (26% lower coronary artery disease and 29% lower heart failure risk).
• Jones, L.W. et al. (2014) Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol 32 (32), 3643–50. Cohort study of nearly 1200 Hodgkin’s lymphoma patients enrolled in the Childhood Cancer Survivorship Study indicating a 51% reduction in cardiovascular events in patients with ≥ 9 metabolic equivalent task [MET]-hours per week. The risk reduction was most notable for coronary artery disease and valvular heart disease, but also included cardiovascular death.
Bergenthal N, et al. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev. 2014;11:CD009075.
Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32(4):335–46.
Velthuis MJ, May AM, Koppejan-Rensenbrink RAG, Gijsen BCM, van Breda E, de Wit GA, et al. Physical Activity during Cancer Treatment (PACT) Study: design of a randomised clinical trial. BMC Cancer. 2010;10:272.
Teleni L, Chan RJ, Chan A, Isenring EA, Vela I, Inder WJ, et al. Exercise improves quality of life in androgen deprivation therapy-treated prostate cancer: systematic review of randomised controlled trials. Endocr Relat Cancer. 2016;23(2):101–12.
MacVicar MG, et al. Effects of aerobic interval training on cancer patients’ functional capacity. Nurs Res. 1989;38(6):348-51.
Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657–65.
Kolden GG, Strauman TJ, Ward A, Kuta J, Woods TE, Schneider KL, et al. A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psychooncology. 2002;11(5):447–56.
Drouin JS, Young TJ, Beeler J, Byrne K, Birk TJ, Hryniuk WM, et al. Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer. 2006;107(10):2490–5.
Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nurs. 2006;29(2):156–65.
Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–404.
Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res. 2009;15(15):4963–7.
Ligibel JA, Partridge A, Giobbie-Hurder A, Campbell N, Shockro L, Salinardi T, et al. Physical and psychological outcomes among women in a telephone-based exercise intervention during adjuvant therapy for early stage breast cancer. J Women’s Health (Larchmt). 2010;19(8):1553–9.
Noble M, Russell C, Kraemer L, Sharratt M. UW WELL-FIT: the impact of supervised exercise programs on physical capacity and quality of life in individuals receiving treatment for cancer. Support Care Cancer. 2012;20(4):865–73.
Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105(23):1821–32.
Vincent F, Labourey JL, Leobon S, Antonini MT, Lavau-Denes S, Tubiana-Mathieu N. Effects of a home-based walking training program on cardiorespiratory fitness in breast cancer patients receiving adjuvant chemotherapy: a pilot study. Eur J Phys Rehabil Med. 2013;49(3):319–29.
Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53(1):65–74.
Travier N, Velthuis MJ, Steins Bisschop CN, van den Buijs B, Monninkhof EM, Backx F, et al. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med. 2015;13:121.
Grabenbauer A, Grabenbauer AJ, Lengenfelder R, Grabenbauer GG, Distel LV. Feasibility of a 12-month-exercise intervention during and after radiation and chemotherapy in cancer patients: impact on quality of life, peak oxygen consumption, and body composition. Radiat Oncol. 2016;11:42.
Cornette T, Vincent F, Mandigout S, Antonini MT, Leobon S, Labrunie A, et al. Effects of home-based exercise training on VO2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (SAPA): a randomized controlled trial. Eur J Phys Rehabil Med. 2016;52(2):223–32.
Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–8.
Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol (1985). 2005;98(4):1534–40.
Hutnick NA, et al. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc. 2005;37(11):1827–35.
Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005;23(15):3577–87.
Daley AJ, Crank H, Mutrie N, Saxton JM, Coleman R. Determinants of adherence to exercise in women treated for breast cancer. Eur J Oncol Nurs. 2007;11(5):392–9.
Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Cancer treatment-induced alterations in muscular fitness and quality of life: the role of exercise training. Ann Oncol. 2007;18(12):1957–62.
Hsieh CC, Sprod LK, Hydock DS, Carter SD, Hayward R, Schneider CM. Effects of a supervised exercise intervention on recovery from treatment regimens in breast cancer survivors. Oncol Nurs Forum. 2008;35(6):909–15.
Casla S, López-Tarruella S, Jerez Y, Marquez-Rodas I, Galvão DA, Newton RU, et al. Supervised physical exercise improves VO2max, quality of life, and health in early stage breast cancer patients: a randomized controlled trial. Breast Cancer Res Treat. 2015;153(2):371–82.
Dolan, L.B. et al. (2017) The cardiac rehabilitation model improves fitness, quality of life, and depression in breast cancer survivors. J Cardiopulm Rehabil Prev.
Bonsignore A, Marzolini S, Oh P. Cardiac rehabilitation for women with breast cancer and treatment-related heart failure compared with coronary artery disease: a retrospective study. J Rehabil Med. 2017;49(3):277–81.
Ligibel JA, Giobbie-Hurder A, Shockro L, Campbell N, Partridge AH, Tolaney SM, et al. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer. 2016;122(8):1169–77.
Lipsett A, Barrett S, Haruna F, Mustian K, O'Donovan A. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast. 2017;32:144–55.
Keats MR, et al. EXercise to prevent AnthrCycline-based Cardio-Toxicity (EXACT) in individuals with breast or hematological cancers: a feasibility study protocol. Pilot Feasibility Stud. 2016;2:44.
Pituskin E, Haykowsky M, McNeely M, Mackey J, Chua N, Paterson I. Rationale and design of the multidisciplinary team IntervenTion in cArdio-oNcology study (TITAN). BMC Cancer. 2016;16(1):733.
Wengstrom Y, et al. Optitrain: a randomised controlled exercise trial for women with breast cancer undergoing chemotherapy. BMC Cancer. 2017;17(1):100.
Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92(8):715–27.
• Gillis, C. et al. (2014) Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 121 (5), 937–47. An important study of 77 colorectal cancer patients undergoing surgery outlining superiority of a before surgery (prehabilitation) versus after surgery (rehabilitation) multimodality exercise and nutrition program in terms of 8-week post 6-min walking distance and recovery to baseline exercise capacity.
Pouwels S, Fiddelaers J, Teijink JAW, Woorst JF, Siebenga J, Smeenk FWJM. Preoperative exercise therapy in lung surgery patients: a systematic review. Respir Med. 2015;109(12):1495–504.
•• Carli F, et al. Surgical prehabilitation in patients with cancer: state-of-the-science and recommendations for future research from a panel of subject ,atter experts. Phys Med Rehabil Clin N Am. 2017;28(1):49–64. A very important state-of-the-art consensus document on the emerging concept of prehabilitation with a summary of past efforts, current stand, and future perspective.
Combs AB, Hudman SL, Bonner HW. Effect of exercise stress upon the acute toxicity of adriamycin in mice. Res Commun Chem Pathol Pharmacol. 1979;23(2):395–8.
Kanter, M.M., Hamlin R.L., Unverferth D.V., Davis H.W., Merola A.J. (1985) Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J Appl Physiol (1985) 59 (4), 1298–1303.
Ji LL, Mitchell EW. Effects of Adriamycin on heart mitochondrial function in rested and exercised rats. Biochem Pharmacol. 1994;47(5):877–85.
Ascensao A, et al. Endurance training attenuates doxorubicin-induced cardiac oxidative damage in mice. Int J Cardiol. 2005;100(3):451–60.
Chicco AJ, Schneider CM, Hayward R. Voluntary exercise protects against acute doxorubicin cardiotoxicity in the isolated perfused rat heart. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R424–31.
Chicco AJ, Schneider CM, Hayward R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovasc Pharmacol. 2006;47(2):182–9.
Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Pöss J, et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J Am Coll Cardiol. 2008;52(6):470–82.
Wonders KY, Hydock DS, Schneider CM, Hayward R. Acute exercise protects against doxorubicin cardiotoxicity. Integr Cancer Ther. 2008;7(3):147–54.
Wonders KY, Hydock DS, Greufe S, Schneider CM, Hayward R. Endurance exercise training preserves cardiac function in rats receiving doxorubicin and the HER-2 inhibitor GW2974. Cancer Chemother Pharmacol. 2009;64(6):1105–13.
Kavazis AN, Smuder AJ, Min K, Tümer N, Powers SK. Short-term exercise training protects against doxorubicin-induced cardiac mitochondrial damage independent of HSP72. Am J Physiol Heart Circ Physiol. 2010;299(5):H1515–24.
Hydock DS, Lien CY, Jensen BT, Schneider CM, Hayward R. Exercise preconditioning provides long-term protection against early chronic doxorubicin cardiotoxicity. Integr Cancer Ther. 2011;10(1):47–57.
Ascensao, A. et al. (2011) Acute exercise protects against calcium-induced cardiac mitochondrial permeability transition pore opening in doxorubicin-treated rats. Clin Sci (Lond) 120 (1), 37–49.
Gibson NM, Greufe SE, Hydock DS, Hayward R. Doxorubicin-induced vascular dysfunction and its attenuation by exercise preconditioning. J Cardiovasc Pharmacol. 2013;62(4):355–60.
Smuder, A.J., Kavazis A.N., Min K., Powers S.K. (2013) Doxorubicin-induced markers of myocardial autophagic signaling in sedentary and exercise trained animals. J Appl Physiol (1985) 115 (2), 176–185.
Jensen BT, Lien CY, Hydock DS, Schneider CM, Hayward R. Exercise mitigates cardiac doxorubicin accumulation and preserves function in the rat. J Cardiovasc Pharmacol. 2013;62(3):263–9.
Parry TL, Hayward R. Exercise training does not affect anthracycline antitumor efficacy while attenuating cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;309(6):R675–83.
Lien CY, Jensen BT, Hydock DS, Hayward R. Short-term exercise training attenuates acute doxorubicin cardiotoxicity. J Physiol Biochem. 2015;71(4):669–78.
Marques-Aleixo I, Santos-Alves E, Mariani D, Rizo-Roca D, Padrão AI, Rocha-Rodrigues S, et al. Physical exercise prior and during treatment reduces sub-chronic doxorubicin-induced mitochondrial toxicity and oxidative stress. Mitochondrion. 2015;20:22–33.
Chicco, A.J., Hydock D.S., Schneider C.M., Hayward R. (2006) Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. J Appl Physiol (1985) 100 (2), 519–527.
Hydock DS, Wonders KY, Schneider CM, Hayward R. Voluntary wheel running in rats receiving doxorubicin: effects on running activity and cardiac myosin heavy chain. Anticancer Res. 2009;29(11):4401–7.
Matsuura C, Brunini TMC, Carvalho LCMM, Resende AC, Carvalho JJ, de Castro JPW, et al. Exercise training in doxorubicin-induced heart failure: effects on the L-arginine-NO pathway and vascular reactivity. J Am Soc Hypertens. 2010;4(1):7–13.
Hydock DS, Lien CY, Jensen BT, Parry TL, Schneider CM, Hayward R. Rehabilitative exercise in a rat model of doxorubicin cardiotoxicity. Exp Biol Med (Maywood). 2012;237(12):1483–92.
Hayward R, Lien CY, Jensen BT, Hydock DS, Schneider CM. Exercise training mitigates anthracycline-induced chronic cardiotoxicity in a juvenile rat model. Pediatr Blood Cancer. 2012;59(1):149–54.
Dolinsky VW, Rogan KJ, Sung MM, Zordoky BN, Haykowsky MJ, Young ME, et al. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. Am J Physiol Endocrinol Metab. 2013;305(2):E243–53.
Sturgeon K, Schadler K, Muthukumaran G, Ding D, Bajulaiye A, Thomas NJ, et al. Concomitant low-dose doxorubicin treatment and exercise. Am J Physiol Regul Integr Comp Physiol. 2014;307(6):R685–92.
Heon S, et al. Dexrazoxane does not protect against doxorubicin-induced damage in young rats. Am J Physiol Heart Circ Physiol. 2003;285(2):H499–506.
Ascensao A, et al. Exercise as a beneficial adjunct therapy during doxorubicin treatment—role of mitochondria in cardioprotection. Int J Cardiol. 2012;156(1):4–10.
Vejpongsa P, Yeh ET. Topoisomerase 2beta: a promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin Pharmacol Ther. 2014;95(1):45–52.
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42.
Powers SK, Smuder AJ, Kavazis AN, Quindry JC. Mechanisms of exercise-induced cardioprotection. Physiology (Bethesda). 2014;29(1):27–38.
Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–26.
Wolin KY, Schwartz AL, Matthews CE, Courneya KS, Schmitz KH. Implementing the exercise guidelines for cancer survivors. J Support Oncol. 2012;10(5):171–7.
Yu AF, Jones LW. Breast cancer treatment-associated cardiovascular toxicity and effects of exercise countermeasures. Cardiooncology. 2016:2.
Gollhofer SM, Wiskemann J, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, et al. Factors influencing participation in a randomized controlled resistance exercise intervention study in breast cancer patients during radiotherapy. BMC Cancer. 2015;15:186.
Courneya KS, Stevinson C, McNeely ML, Sellar CM, Peddle CJ, Friedenreich CM, et al. Predictors of adherence to supervised exercise in lymphoma patients participating in a randomized controlled trial. Ann Behav Med. 2010;40(1):30–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ray W. Squires, Adam M. Shultz, and Joerg Herrmann declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Squires, R.W., Shultz, A.M. & Herrmann, J. Exercise Training and Cardiovascular Health in Cancer Patients. Curr Oncol Rep 20, 27 (2018). https://doi.org/10.1007/s11912-018-0681-2
Published:
DOI: https://doi.org/10.1007/s11912-018-0681-2