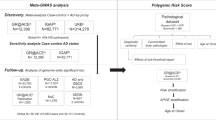
Abstract
Clinical trials for Alzheimer’s disease are now focusing on the earliest stages of the disease with the goal of delaying dementia onset. There is great utility in using genetic variants to identify individuals at high age-dependent risk when the goal is to begin treatment before the development of any cognitive symptoms. Genetic variants identified through large-scale genome-wide association studies have not substantially improved the accuracy provided by APOE genotype to identify people at high risk of late-onset Alzheimer’s disease (LOAD). We describe novel approaches, focused on molecular phylogenetics, to finding genetic variants that predict age at LOAD onset with sufficient accuracy and precision to be useful. We highlight the discovery of a polymorphism in TOMM40 that, in addition to APOE, may improve risk prediction and review how TOMM40 genetic variants may impact the develop of LOAD independently from APOE. The analysis methods described in this review may be useful for other genetically complex human diseases.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(4):37.
Mayeux R et al. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment-Part 1. Alzheimers Dement. 2011;7(1):15–34.
Albert MS et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9.
Reiman, E.M., et al. CAP-advancing the evaluation of preclinical Alzheimer disease treatments. Nat Rev Neurol. 2016;12(1):56–61.
Pericak-Vance MA et al. Linkage studies in familial Alzheimer disease: evidence for chromosome 19 linkage. Am J Hum Genet. 1991;48(6):1034–50.
Corder EH et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3.
Liu N et al. Haplotype-Association Analysis. Adv Genet. 2008;60:335–405.
Schmechel DE et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci. 1993;90(20):9649–53.
Saunders AM et al. Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–72.
Medway C, Morgan K. Review: the genetics of Alzheimer’s disease; putting flesh on the bones. Neuropathol Appl Neurobiol. 2014;40(2):97–105.
Lambert J-C et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–8.
Chouraki, V. and S. Seshadri, Genetics of Alzheimer’s disease. Adv Genet, 2014. 87: p. 245-94. This is a comprehensive review of the genetics of Alzheimer’s Disease that includes relevant discussion about structural variants, rare variants, untangling the genetics of the APOE-TOMM40 region and gene-environment interactions. Translational genetics are addressed with emphasis on using genetics for risk prediction and for identification of new drug targets.
Hollingworth P et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429-35.
Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360(17):1696–8.
Ridge PG et al. Alzheimer’s disease: analyzing the missing heritability. PLoS One. 2013;8(11):e79771.
Gatz M et al. Role of Genes and Environments for Explaining Alzheimer Disease. Arch Gen Psychiatry. 2006;63(2):168–74.
De Stefano A, S.S, Fitzpatrick A, Ikram M, Gudnason V, Boada M, et al. Genome-wide association studies using prospective cohorts: the CHARGE consortium. Alzheimers Dement. 2011;7(4):S480.
Harold D et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1088–93.
MacArthur DG et al. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508(7497):469–76.
Zhu Q et al. Prioritizing genetic variants for causality on the basis of preferential linkage disequilibrium. Am J Hum Genet. 2012;91(3):422–34.
Bochdanovits Z et al. Accurate prediction of a minimal region around a genetic association signal that contains the causal variant. Eur J Hum Genet. 2014;22(2):238–42.
Teo YY et al. Identifying candidate causal variants via trans-population fine-mapping. Genet Epidemiol. 2010;34(7):653–64.
Liu Y et al. A regression-based association test for case-control studies that uses inferred ancestral haplotype similarity. Ann Hum Genet. 2009;73(Pt 5):520–6.
Roses AD et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2010;10(5):375–84.
Liu Y et al. IL-6 haplotypes, inflammation, and risk for cardiovascular disease in a multiethnic dialysis cohort. J Am Soc Nephrol. 2006;17(3):863–70.
Vardarajan BN et al. Coding mutations in SORL1 and Alzheimer disease. Ann Neurol. 2015;77(2):215–27.
Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009;50(Suppl):S183–8.
Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:3.
Linnertz C, A.L., Gottschalk W, Crenshaw D, Lutz MW, Allen J, Saith S, Mihovilovic M, Burke JR, Welsh-Bohmer KA, Roses AD, Chiba-Falek O, The cis-regulatory effect of an Alzheimer’s disease-associated poly-T locus on expression of TOMM40 and APOE genes. Alzheimers & Dement. 2014;10(5):541–51.
Allen M et al. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology. 2012;79(3):221–8.
Roses AD et al. New applications of disease genetics and pharmacogenetics to drug development. Curr Opin Pharmacol. 2014;14:81–9.
Cruchaga C et al. Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families. PLoS One. 2012;7(2):e31039.
Sassi C et al. Investigating the role of rare coding variability in Mendelian dementia genes (APP, PSEN1, PSEN2, GRN, MAPT, and PRNP) in late-onset Alzheimer’s disease. Neurobiol Aging. 2014;35(12):2881. e1-6.
Jonsson T et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488(7409):96–9.
Medway CW et al. ApoE variant p.V236E is associated with markedly reduced risk of Alzheimer’s disease. Mol Neurodegener. 2014;9:11.
Lord J, Lu AJ, Cruchaga C. Identification of rare variants in Alzheimer’s disease. Front Genet. 2014;5:369.
Ringman J et al. Genetic Heterogeneity in Alzheimer Disease and Implications for Treatment Strategies. Curr Neurol Neurosci Rep. 2014;14(11):1–9. This study advanced the idea that AD is heterogeneous and that different genetic subtypes of the disease may not uniformly respond to a given intervention. Examples from the recently failed AD therapeutic trials are shared to illustrate the importance of considering biologic pathway differences associated with different genetic origins when designing clinical trials.
Beckett LA et al. The Alzheimer’s disease neuroimaging initiative phase 2: increasing the length, breadth, and depth of our understanding. Alzheimers Dement. 2015;11(7):823–31.
Nettiksimmons J et al. Biological heterogeneity in ADNI amnestic mild cognitive impairment. Alzheimers Dement. 2014;10(5):511–21. e1.
Browning S. Missing data imputation and haplotype phase inference for genome-wide association studies. Hum Genet. 2008;124(5):439–50.
Schaid DJ. Evaluating associations of haplotypes with traits. Genet Epidemiol. 2004;27(4):348–64.
Bader JS. The relative power of SNPs and haplotype as genetic markers for association tests. Pharmacogenomics. 2001;2(1):11–24.
Akey J, Jin L, Xiong M. Haplotypes vs single marker linkage disequilibrium tests: what do we gain? Eur J Hum Genet. 2001;9(4):291–300.
Morris RW, Kaplan NL. On the advantage of haplotype analysis in the presence of multiple disease susceptibility alleles. Genet Epidemiol. 2002;23(3):221–33.
Raelson JV et al. Genome-wide association study for Crohn’s disease in the Quebec Founder Population identifies multiple validated disease loci. Proc Natl Acad Sci U S A. 2007;104(37):14747–52.
Lee JH et al. A haplotype-based molecular analysis of CFTR mutations associated with respiratory and pancreatic diseases. Hum Mol Genet. 2003;12(18):2321–32.
Bekris LM et al. APOE mRNA and protein expression in postmortem brain are modulated by an extended haplotype structure. Am J Med Genet Part B. 2010;153B(2):409–17.
Tan EK et al. SORL1 haplotypes modulate risk of Alzheimer’s disease in Chinese. Neurobiol Aging. 2009;30(7):1048–51.
Reitz C et al. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol. 2011;68(1):99–106.
Templeton AR, Crandall KA, Sing CF. A Cladistic Analysis of Phenotypic Associations With Haplotypes Inferred From Restriction Endonuclease Mapping and DNA Sequence Data, III Cladogram Estimation. Genetics. 1992;132(2):619–33.
Clark TG, De Iorio M, Griffiths RC. Bayesian logistic regression using a perfect phylogeny. Biostatistics. 2007;8(1):32–52.
Minichiello MJ, Durbin R. Mapping trait loci by use of inferred ancestral recombination graphs. Am J Hum Genet. 2006;79(5):910–22.
Morris AP, Whittaker JC, Balding DJ. Fine-scale mapping of disease loci via shattered coalescent modeling of genealogies. AmJ Hum Genet. 2002;70(3):686–707.
Zöllner S, Pritchard JK. Coalescent-based association mapping and fine mapping of complex trait loci. Genetics. 2005;169(2):1071–92.
Bardel C et al. On the use of haplotype phylogeny to detect disease susceptibility loci. BMC Genet. 2005;6(1):24–37.
Ding ZH, Mailund T, Song YS. Efficient whole-genome association mapping using local phylogenies for unphased genotype data. Bioinformatics. 2008;24(19):2215–21.
Durrant C et al. Linkage disequilibrium mapping via cladistic analysis of single-nucleotide polymorphism haplotypes. Am J Hum Genet. 2004;75(1):35–43.
Liu J, Papasian C, Deng HW. Incorporating single-locus tests into haplotype cladistic analysis in case-control studies. PLoS Genet. 2007;3(3):e46.
Mailund T, Besenbacher S, Schierup MH. Whole genome association mapping by incompatibilities and local perfect phylogenies. BMC Bioinformatics. 2006;7:22.
Seltman H, Roeder K, Devlin B. Evolutionary-based association analysis using haplotype data. Genet Epidemiol. 2003;25(1):48–58.
Sevon P, Toivonen H, Ollikainen V. TreeDT: tree pattern mining for gene mapping. IEEE/ACM Trans Comput Biol Bioinform. 2006;3(2):174–85.
Su SY, Balding DJ, Coin LJ. Disease association tests by inferring ancestral haplotypes using a hidden markov model. Bioinformatics. 2008;24(7):972–8.
Tachmazidou I, Verzilli C, Iorio M. Genetic association mapping via evolution-based clustering of haplotypes. PLoS Genet. 2007;3(7):e111.
Thomas DC et al. Bayesian spatial modeling of haplotype associations. Hum Hered. 2003;56(1-3):32–40.
Tzeng JY. Evolutionary-based grouping of haplotypes in association analysis. Genet Epidemiol. 2005;28(3):220–31.
Waldron ER, Whittaker JC, Balding DJ. Fine mapping of disease genes via haplotype clustering. Genet Epidemiol. 2006;30(2):170–9.
Templeton AR. The diverse applications of cladistic analysis of molecular evolution, with special reference to nested clade analysis. Int J Mol Sci. 2010;11(1):124–39. Templeton describes the application of evolutionary genealogy of DNA lineages, including intraspecific studies, for genotype/phenotype associations at candidate loci and in genome-wide association studies. The author also establishes the theoretical framework of using phylogenetic analysis to untangle a region of high linkage disequilibrium for a genotype/phenotype association study.
Browning BL, Browning SR. Haplotypic analysis of Wellcome Trust Case Control Consortium data. Hum Genet. 2008;123(3):273–80.
Browning, S.R. and B.L. Browning, Haplotype phasing: existing methods and new developments. Nat Rev Genet. 2011;12(10):703–14
Crenshaw DG et al. Using genetics to enable studies on the prevention of Alzheimer’s disease. Clin Pharmacol Ther. 2013;93(2):177–85. This paper presented the use of a composite genetic biomarker to stratify for disease risk in clinical studies aimed at delaying or preventing the onset of AD in individuals at high risk.
Roses AD et al. TOMM40 and APOE: requirements for replication studies of association with age of disease onset and enrichment of a clinical trial. Alzheimers Dement. 2013;9(2):132–6.
Johnson SC et al. The effect of TOMM40 poly-T length on gray matter volume and cognition in middle-aged persons with APOE ɛ3/ɛ3 genotype. Alzheimer’s Dementia. 2011;7(4):456–65.
Hayden KM et al. A homopolymer polymorphism in the TOMM40 gene contributes to cognitive performance in aging. Alzheimers Dement. 2012;8(5):381–8.
Huson DH, Scornavacca C. A survey of combinatorial methods for phylogenetic networks. Genome Biol Evol. 2011;3:23–35.
Tewhey R et al. The importance of phase information for human genomics. Nat Rev Genet. 2011;12(3):215–23.
Dickson SP et al. Rare variants create synthetic genome-wide associations. PLoS Biol. 2010;8(1), e1000294.
Lescai, F., et al., An APOE haplotype associated with decreased epsilon4 expression increases the risk of late onset Alzheimer’s disease. J Alzheimers Dis. 2011;24(2):235–45.
Hendrie HC et al. Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA. 2001;285(6):739–47.
Hendrie HC et al. Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152(10):1485–92.
Hall KS et al. Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimers Dement. 2009;5(3):227–33.
Tang MX et al. Relative risk of Alzheimer disease and age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet. 1996;58(3):574–84.
Tang MX et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56.
Roses AD et al. African-American TOMM40’523-APOE haplotypes are admixture of West African and Caucasian alleles. Alzheimers Dement. 2014;10(6):592–601. e2.
Bekris LM, Lutz F, Yu C-E. Functional analysis of APOE locus genetic variation implicates regional enhancers in the regulation of both TOMM40 and APOE. J Hum Genet. 2012;57(1):18–25.
Jun G et al. Comprehensive search for Alzheimer disease susceptibility loci in the APOE region. Arch Neurol. 2012;69(10):1270–9.
Cruchaga C et al. Association and expression analyses with single-nucleotide polymorphisms in TOMM40 in Alzheimer disease. Arch Neurol. 2011;68(8):1013–9.
Li G et al. TOMM40 intron 6 poly-T length, age at onset, and neuropathology of AD in individuals with APOE epsilon3/epsilon3. Alzheimers Dement. 2013;9(5):554–61.
Akai J, Kimura A, Hata RI. Transcriptional regulation of the human type I collagen alpha2 (COL1A2) gene by the combination of two dinucleotide repeats. Gene. 1999;239(1):65–73.
Chiba-Falek O, Nussbaum RL. Effect of allelic variation at the NACP-Rep1 repeat upstream of the alpha-synuclein gene (SNCA) on transcription in a cell culture luciferase reporter system. Hum Mol Genet. 2001;10(26):3101–9.
Okladnova O et al. A promoter-associated polymorphic repeat modulates PAX-6 expression in human brain. Biochem Biophys Res Commun. 1998;248(2):402–5.
Peters DG et al. Functional polymorphism in the matrix metalloproteinase-9 promoter as a potential risk factor for intracranial aneurysm. Stroke. 1999;30(12):2612–6.
Searle S, Blackwell JM. Evidence for a functional repeat polymorphism in the promoter of the human NRAMP1 gene that correlates with autoimmune versus infectious disease susceptibility. J Med Genet. 1999;36(4):295–9.
Shimajiri S et al. Shortened microsatellite d(CA)21 sequence down-regulates promoter activity of matrix metalloproteinase 9 gene. FEBS Lett. 1999;455(1-2):70–4.
Hefferon TW et al. A variable dinucleotide repeat in the CFTR gene contributes to phenotype diversity by forming RNA secondary structures that alter splicing. Proc Natl Acad Sci U S A. 2004;101(10):3504–9.
Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447(7147):932–40.
Pearson CE, Nichol Edamura K, Cleary JD. Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet. 2005;6(10):729–42.
Willems T et al. The landscape of human STR variation. Genome Res. 2014;24(11):1894–904.
Sudmant PH et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526(7571):75–81.
Linnertz C et al. Characterization of the poly-T variant in the TOMM40 gene in diverse populations. PLoS One. 2012;7(2):e30994.
Gottschalk WK et al. The Broad Impact of TOM40 on Neurodegenerative Diseases in Aging. J Parkinson’s Dis Alzheimer’s Dis. 2014;1(1):12. This article summarizes the molecular and genetic evidence relating Tom40 protein and the Translocase of the Outer Mitochondrial Membrane complex to mitochondrial dysfunction and neurological disease, including LOAD and Parkinson’s disease.
Bender A et al. TOM40 mediates mitochondrial dysfunction induced by alpha-synuclein accumulation in Parkinson’s disease. PLoS One. 2013;8(4):e62277.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Michael Lutz has received a grant from NIA/NIH and personal fees from Zinfandel Pharmaceuticals.
Donna Crenshaw is a paid consultant to Zinfandel Pharmaceuticals.
Kathleen Welsh-Bohmer has received a grant from Takeda Pharmaceutical Company.
Daniel K. Burns is an employee of Zinfandel Pharmaceuticals.
Allen D. Roses is sole shareholder of NC S-Corp. (Zinfandel Pharmaceuticals, Inc.)
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Dementia
Rights and permissions
About this article
Cite this article
Lutz, M.W., Crenshaw, D., Welsh-Bohmer, K.A. et al. New Genetic Approaches to AD: Lessons from APOE-TOMM40 Phylogenetics. Curr Neurol Neurosci Rep 16, 48 (2016). https://doi.org/10.1007/s11910-016-0643-8
Published:
DOI: https://doi.org/10.1007/s11910-016-0643-8