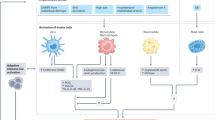
Abstract
Purpose of Review
Despite enhanced screening and therapeutic management, hypertension remains the most prevalent chronic disease in the United States and the leading cause of heart disease, chronic kidney disease, and stroke in both men and women. It is widely accepted that hypertension is a pro-inflammatory disease and that the immune system plays a vital role in mediating hypertensive outcomes and end organ damage. Despite known discrepancies in the risk of hypertension development between men and women, preclinical models of immune-mediated hypertension were historically developed solely in male animals, leading to a lack of sex-specific clinical practice guidelines or therapeutic targets.
Recent Findings
Following the NIH policy on the consideration of sex as a biological variable in 2015, significant advancements have been made into sex-specific disease mechanisms in inflammation and hypertension.
Summary
This review article serves to critically evaluate recent advancements in the field of sex-specific immune-mediated hypertension.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Heart Disease and Stroke Statistics—2015. Update: a report from the American Heart Association. Circulation. 2015;131:e29–322.
Heart Disease and Stroke Statistics—2019. Update: a report from the American Heart Association. Circulation. 2019;139:e56–528.
Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–33.
Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, et al. Hypertension and its treatment in postmenopausal women: baseline data from the women’s health initiative. Hypertension. 2000;36:780–9.
Nwankwo T, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013;133:1–8.
Rodriguez-Iturbe B, Pons H, Johnson RJ. Role of the immune system in hypertension. Physiol Rev. 2017;97:1127–64.
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–60.
•• Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, et al. sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–90 First identification of a sex difference in T cell-mediated hypertension using male and female Rag-1 −/− mouse model.
Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, et al. Sex-specific T-cell regulation of angiotensin II–dependent hypertension novelty and significance. Hypertension. 2014;64:573–82.
Peeters AC, Netea MG, Janssen MC, Kullberg BJ, Van der Meer JW, Thien T. Pro-inflammatory cytokines in patients with essential hypertension. Eur J Clin Investig. 2001;31:31–6.
McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–33.
Norlander AE, Madhur MS. Inflammatory cytokines regulate renal sodium transporters: how, where, and why? Am J Physiol Ren Physiol. 2017;313:F141–4.
Barbaro NR, Harrison DG. Markers or makers: inflammatory cytokines in treatment-resistant hypertension. Hypertension. 2019;73:767–9.
Lee DL, Sturgis LC, Labazi H, Osborne JB, Fleming C, Pollock JS, et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–40.
Li K, Guo D, Zhu H, Hering-Smith KS, Hamm LL, Ouyang J, et al. Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am J Phys Regul Integr Comp Phys. 2010;299:R590–5.
Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Ren Physiol. 2016;311:F555–61.
O’Leary R, Penrose H, Miyata K, Satou R. Macrophage-derived IL-6 contributes to ANG II-mediated angiotensinogen stimulation in renal proximal tubular cells. Am J Physiol Ren Physiol. 2016;310:F1000–7.
• Itani HA, McMaster WG, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba A, et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. 2016;68:123–32 First known use of humanized mice to study immune-mediated hypertension. Humanized mice were used to study the impact of human T cell activation on hypertensive outcomes specifically in male mice.
Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM. Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res. 2013;97:696–704.
Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–7.
Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension. 2016;68:167–74.
Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ−/− and interleukin-17A−/− mice. Hypertension. 2015;65:569–76.
Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, et al. T regulatory lymphocytes prevent angiotensin II–induced hypertension and vascular injury. Hypertension. 2011;57:469–76.
Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Phys Regul Integr Comp Phys. 2012;303:R359–67.
•• Tipton AJ, Musall JB, Crislip GR, Sullivan JC. Greater transforming growth factor-β in adult female SHR is dependent on blood pressure, but does not account for sex differences in renal T-regulatory cells. Am J Physiol Ren Physiol. 2017;313:F847–53 Important investigation into sex-specific TGF-β signaling and sex difference in T regulatory cell expression in spontaneously hypertensive rats.
Taylor LE, Gillis EE, Musall JB, Baban B, Sullivan JC. High-fat diet-induced hypertension is associated with a proinflammatory T cell profile in male and female Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol. 2018;315:H1713–23.
Tamaya-Mori N, Uemura K, Iguchi A. Gender differences in the dietary lard-induced increase in blood pressure in rats. Hypertension. 2002;39:1015–20.
Roberts CK, Vaziri ND, Barnard RJ. Protective effects of estrogen on gender-specific development of diet-induced hypertension. J Appl Physiol. 2001;91:2005–9.
•• Pollow, DP, Uhlorn, J, Sylvester, MA, Romero-Aleshire, MJ, Uhrlaub, JU, Lindsey, M, et al. Menopause and FOXP3+ Treg cell depletion eliminate female protection against T cell-mediated Angiotensin II hypertension. Am J Physiol Heart Circ Physiol. 2019; in press Depletion of FoxP3 + T regulatory cells eliminates premenopausal protection from hypertension.
Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7.
• Norlander AE, Saleh MA, Pandey AK, Itani HA, Wu J, Xiao L, et al. A salt-sensing kinase in T lymphocytes, SGK1, drives hypertension and hypertensive end-organ damage. JCI Insight. 2017;2:13 In males, evidence of salt-sensing signaling on T cell leads to hypertensive response.
Du Y-N, Tang X-F, Xu L, Chen W-D, Gao P-J, Han W-Q. SGK1-FoxO1 signaling pathway mediates Th17/Treg imbalance and target organ inflammation in angiotensin II-induced hypertension. Front Physiol. 2018;9:1581.
Sun X-N, Li C, Liu Y, Du L-J, Zeng M-R, Zheng X-J, et al. T-cell mineralocorticoid receptor controls blood pressure by regulating interferon-gamma. Circ Res. 2017;120:1584–97.
Maas AHEM, Franke HR. Women’s health in menopause with a focus on hypertension. Neth Hear J. 2009;17:68–72.
Vural P, Akgul C, Canbaz M. Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol Res. 2006;54:298–302.
Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40:66–73.
Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–13.
Lélu K, Laffont S, Delpy L, Paulet P-E, Périnat T, Tschanz SA, et al. Estrogen receptor α signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2386–93.
Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol. 2007;292:H1770–6.
•• Pollow DP, Romero-Aleshire MJ, Sanchez JN, Konhilas JP, Brooks HL. ANG II-induced hypertension in the VCD mouse model of menopause is prevented by estrogen replacement during perimenopause. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1546–52 First evidence that female mice lose protection from hypertension following ovarian failure using the VCD model of menopause.
Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, et al. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res. 2016;118:1233–43.
• Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017;21:1009–20 Evidence of sodium signaling directly on dendritic cells leading to increased hypertensive response specifically in male mice.
Cheng H-F, Harris RC. Cyclooxygenases, the kidney, and hypertension. Hypertension. 2004;43:525–30.
D’Abril Ruíz-Leyja E, Villalobos-Molina R, López-Guerrero JJ, Gallardo-Ortíz IA, Estrada-Soto SE, Ibarra-Barajas M. Differential role of cyclooxygenase-1 and -2 on renal vasoconstriction to α1-adrenoceptor stimulation in normotensive and hypertensive rats. Life Sci. 2013;93:552–7.
Sullivan JC, Sasser JM, Pollock DM, Pollock JS. Sexual dimorphism in renal production of Prostanoids in spontaneously hypertensive rats. Hypertension. 2005;45:406–11.
• Elmarakby AA, Katary M, Pollock JS, Sullivan JC. Influence of the selective COX-2 inhibitor celecoxib on sex differences in blood pressure and albuminuria in spontaneously hypertensive rats. Prostaglandins Other Lipid Mediat. 2018;135:16–20 First investigation into COX-2 signaling mechanisms in both male and female spontaneously hypertensive rats.
Lebreton F, Berishvili E, Parnaud G, Rouget C, Bosco D, Berney T, et al. NLRP3 inflammasome is expressed and regulated in human islets. Cell Death Dis. 2018;9:726.
Yin J, Zhao F, Chojnacki JE, Fulp J, Klein WL, Zhang S, et al. NLRP3 Inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s Disease. Mol Neurobiol. 2018;55:1977–87.
Keane RW, Dietrich WD, de Rivero Vaccari JP. Inflammasome proteins as biomarkers of multiple sclerosis. Front Neurol. 2018;9:135.
Wang Q, So A, Nussberger J, Ives A, Bagnoud N. Renin-dependent hypertension in mice requires the NLRP3- inflammasome. J Hypertens. 2013;3:187.
• Krishnan SM, Ling YH, Huuskes BM, Ferens DM, Saini N, Chan CT, et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc Res. 2019;115:776–87 Novel investigation into the therapeutic potential of the NLRP3 inflammasome pathway in male mice.
Sun H-J, Ren X-S, Xiong X-Q, Chen Y-Z, Zhao M-X, Wang J-J, et al. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 2017;8:e3074.
Varghese GP, Fransén K, Hurtig-Wennlöf A, Bengtsson T, Jansson J-H, Sirsjö A. Q705K variant in NLRP3 gene confers protection against myocardial infarction in female individuals. Biomed Rep. 2013;1:879–82.
Stødle GS, Silva GB, Tangerås LH, Gierman LM, Nervik I, Dahlberg UE, et al. Placental inflammation in pre-eclampsia by nod-like receptor protein (NLRP)3 inflammasome activation in trophoblasts. Clin Exp Immunol. 2018;193:84–94.
Weel I C, Romão-Veiga M, Matias ML, Fioratti EG, Peraçoli JC, Borges VT, et al. Increased expression of NLRP3 inflammasome in placentas from pregnant women with severe preeclampsia. J Reprod Immunol. 2017;123:40–7.
Shirasuna K, Karasawa T, Usui F, Kobayashi M, Komada T, Kimura H, et al. NLRP3 deficiency improves angiotensin II-induced hypertension but not fetal growth restriction during pregnancy. Endocrinology. 2015;156:4281–92.
Funding
This work was funded by the National Institutes of Health Grants HL131834 (H.L.B) and T32HL007249 (M.S.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Inflammation and Cardiovascular Diseases
Rights and permissions
About this article
Cite this article
Sylvester, M.A., Brooks, H.L. Sex-Specific Mechanisms in Inflammation and Hypertension. Curr Hypertens Rep 21, 53 (2019). https://doi.org/10.1007/s11906-019-0959-2
Published:
DOI: https://doi.org/10.1007/s11906-019-0959-2