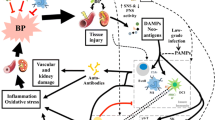
Abstract
Purpose of Review
To describe the important role played by innate and innate-like immunity in the pathophysiology of hypertension and vascular injury.
Recent Findings
Innate immune cells, such as neutrophils, dendritic cells, myeloid-derived suppressor cells, and monocytes/macrophages and innate lymphoid cells such as natural killer cells and unconventional T lymphocytes like γδ T cells contribute to hypertensive mechanisms by priming adaptive immune cells, leading to the triggering of vascular inflammation and blood pressure elevation or alternatively protecting against vascular injury. Specifically, monocyte/macrophages and γδ T cells seem to play a crucial role in the initiation of hypertension via regulation of adaptive immunity.
Summary
Innate and innate-like immunity play a leading role in the pathophysiology of hypertension. Recent advances in this field provide us clues for future therapeutic approaches.

Similar content being viewed by others
Abbreviations
- B7:
-
Co-stimulatory molecule on antigen-presenting cell surface (CD80 or CD86)
- CD28:
-
Cluster of differentiation 28
- CNS:
-
Central nervous system
- MHC II:
-
Major histocompatibility complex II
- TCR:
-
T cell receptor
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215(1):21–33. https://doi.org/10.1084/jem.20171773.
•• Caillon A, Mian MOR, Fraulob-Aquino JC, Huo KG, Barhoumi T, Ouerd S, et al. Gammadelta T cells mediate angiotensin II-induced hypertension and vascular injury. Circulation. 2017;135(22):2155–62. https://doi.org/10.1161/CIRCULATIONAHA.116.027058 This study is the first to point out the role of γδ T cells during hypertension. In this study, a crucial role of γδ T cells on the vascular injury associated with hypertension was observed. Other T cells were not activated in absence of γδ T cells, suggesting that γδ T cells play a role in triggering inflammation in the initiation of hypertension.
Janeway CAJ, Travers P, Walport M, Shlomchik MJ. Immunobiology. 5th ed. New York and London: Garland Science; 2001.
Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. https://doi.org/10.1038/cr.2009.139.
Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–47. https://doi.org/10.1038/nri.2017.105.
Bao X, Meng G, Zhang Q, Liu L, Wu H, Du H, et al. Elevated serum complement C3 levels are associated with prehypertension in an adult population. Clin Exp Hypertens. 2017;39(1):42–9. https://doi.org/10.1080/10641963.2016.1210622.
Engstrom G, Hedblad B, Berglund G, Janzon L, Lindgarde F. Plasma levels of complement C3 is associated with development of hypertension: a longitudinal cohort study. J Hum Hypertens. 2007;21(4):276–82. https://doi.org/10.1038/sj.jhh.1002129.
Martin-Lorenzo M, Gonzalez-Calero L, Martinez PJ, Baldan-Martin M, Lopez JA, Ruiz-Hurtado G, et al. Immune system deregulation in hypertensive patients chronically RAS suppressed developing albuminuria. Sci Rep. 2017;7(1):8894. https://doi.org/10.1038/s41598-017-09042-2.
Cui J, Wan J, You D, Zou Z, Chen Y, Li Z, et al. Interstitial complement C3 activation and macrophage infiltration in patients with hypertensive nephropathy. Clin Nephrol. 2017;88(12):328–37. https://doi.org/10.5414/CN109154.
Zhang C, Li Y, Wang C, Wu Y, Cui W, Miwa T, et al. Complement 5a receptor mediates angiotensin II-induced cardiac inflammation and remodeling. Arterioscler Thromb Vasc Biol. 2014;34(6):1240–8. https://doi.org/10.1161/ATVBAHA.113.303120.
• Chen XH, Ruan CC, Ge Q, Ma Y, Xu JZ, Zhang ZB, et al. Deficiency of complement C3a and C5a receptors orevents angiotensin II-induced hypertension via regulatory T cells. Circ Res. 2018;122(7):970–83. https://doi.org/10.1161/CIRCRESAHA.117.312153 This study suggests an interesting role of complement components in hypertension leading to inhibition of anti-inflammatory cell such as Tregs. This paper was the first to describe the effect of complement depletion on Tregs in the pathophysiology of hypertension. The authors reported that the depletion of both C3aR (complement c3a receptor) and C5aR stimulated Foxp3 expression in vitro and in vivo and prevented the BP elevation induced by Ang II. Their study is noteworthy because it may show an alternative novel approach for hypertension treatment.
Liu X, Zhang Q, Wu H, Du H, Liu L, Shi H, et al. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens. 2015;28(11):1339–46. https://doi.org/10.1093/ajh/hpv034.
Zhang R, Inagawa H, Kazumura K, Tsuchiya H, Miwa T, Morishita N, et al. Evaluation of a hypertensive rat model using peripheral blood neutrophil activity, phagocytic activity and oxidized LDL evaluation. Anticancer Res. 2018;38(7):4289–94. https://doi.org/10.21873/anticanres.12726.
Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. https://doi.org/10.3389/fphys.2018.00113.
De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25(10):2106–13. https://doi.org/10.1161/01.ATV.0000181743.28028.57.
Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, et al. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol. 2007;292(4):H1789–95. https://doi.org/10.1152/ajpheart.01118.2006.
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, et al. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124(12):1370–81. https://doi.org/10.1161/CIRCULATIONAHA.111.034470.
Ishibashi M, Hiasa K, Zhao Q, Inoue S, Ohtani K, Kitamoto S, et al. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004;94(9):1203–10. https://doi.org/10.1161/01.RES.0000126924.23467.A3.
Bush E, Maeda N, Kuziel WA, Dawson TC, Wilcox JN, DeLeon H, et al. CC chemokine receptor 2 is required for macrophage infiltration and vascular hypertrophy in angiotensin II-induced hypertension. Hypertension. 2000;36(3):360–3.
Liao TD, Yang XP, Liu YH, Shesely EG, Cavasin MA, Kuziel WA, et al. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension. 2008;52(2):256–63. https://doi.org/10.1161/HYPERTENSIONAHA.108.112706.
Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJ, Dorweiler B, et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. 2017;127(2):564–8. https://doi.org/10.1172/JCI90520.
DeBerge M, Yeap XY, Dehn S, Zhang S, Grigoryeva L, Misener S, et al. MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ Res. 2017;121(8):930–40. https://doi.org/10.1161/CIRCRESAHA.117.311327.
Harwani SC. Macrophages under pressure: the role of macrophage polarization in hypertension. Transl Res. 2018;191:45–63. https://doi.org/10.1016/j.trsl.2017.10.011.
Kishi T. Regulation of the sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 Academic Conference Award from the Japanese Society of Hypertension. Hypertens Res. 2013;36(10):845–51. https://doi.org/10.1038/hr.2013.73.
Hong MN, Li XD, Chen DR, Ruan CC, Xu JZ, Chen J, et al. Renal denervation attenuates aldosterone expression and associated cardiovascular pathophysiology in angiotensin II-induced hypertension. Oncotarget. 2016;7(42):67828–40. https://doi.org/10.18632/oncotarget.12182.
Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res. 2015;117(6):547–57. https://doi.org/10.1161/CIRCRESAHA.115.306010.
Zaldivia MT, Rivera J, Hering D, Marusic P, Sata Y, Lim B, et al. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension. 2017;69(2):323–31. https://doi.org/10.1161/HYPERTENSIONAHA.116.08373.
• Carnevale D, Perrotta M, Pallante F, Fardella V, Iacobucci R, Fardella S, et al. A cholinergic-sympathetic pathway primes immunity in hypertension and mediates brain-to-spleen communication. Nat Commun. 2016;7:13035. https://doi.org/10.1038/ncomms13035 This study is one of the first describing how the CNS affects egression of immune cells from lymphatic organs. This observation suggests an interesting role of the sympathetic system in the inflammatory process in hypertension.
Harwani SC, Ratcliff J, Sutterwala FS, Ballas ZK, Meyerholz DK, Chapleau MW, et al. Nicotine mediates CD161a+ renal macrophage infiltration and premature hypertension in the spontaneously hypertensive rat. Circ Res. 2016;119(10):1101–15. https://doi.org/10.1161/CIRCRESAHA.116.309402.
Jun JY, Zubcevic J, Qi Y, Afzal A, Carvajal JM, Thinschmidt JS, et al. Brain-mediated dysregulation of the bone marrow activity in angiotensin II-induced hypertension. Hypertension. 2012;60(5):1316–23. https://doi.org/10.1161/HYPERTENSIONAHA.112.199547.
Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, et al. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56(2):297–303. https://doi.org/10.1161/HYPERTENSIONAHA.110.150409.
Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, et al. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66(2):309–16. https://doi.org/10.1161/HYPERTENSIONAHA.115.05333.
Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15(5):545–52. https://doi.org/10.1038/nm.1960.
Choi SY, Lee HH, Lee JH, Ye BJ, Yoo EJ, Kang HJ, et al. TonEBP suppresses IL-10-mediated immunomodulation. Sci Rep. 2016;6:25726. https://doi.org/10.1038/srep25726.
Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551(7682):585–9. https://doi.org/10.1038/nature24628.
Afsar B, Kuwabara M, Ortiz A, Yerlikaya A, Siriopol D, Covic A, et al. Salt intake and immunity. Hypertension. 2018;72(1):19–23. https://doi.org/10.1161/HYPERTENSIONAHA.118.11128.
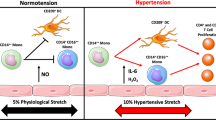
Loperena R, Van Beusecum JP, Itani HA, Engel N, Laroumanie F, Xiao L, et al. Hypertension and increased endothelial mechanical stretch promote monocyte differentiation and activation: roles of STAT3, interleukin 6 and hydrogen peroxide. Cardiovasc Res. 2018;114(11):1547–63. https://doi.org/10.1093/cvr/cvy112.
Paradis P, Schiffrin EL. CXCL1-CXCR2 lead monocytes to the heart of the matter. Eur Heart J. 2018;39(20):1832–4. https://doi.org/10.1093/eurheartj/ehy114.
Wang L, Zhao XC, Cui W, Ma YQ, Ren HL, Zhou X, et al. Genetic and pharmacologic inhibition of the chemokine receptor CXCR2 prevents experimental hypertension and vascular dysfunction. Circulation. 2016;134(18):1353–68. https://doi.org/10.1161/CIRCULATIONAHA.115.020754.
Wang L, Zhang YL, Lin QY, Liu Y, Guan XM, Ma XL, et al. CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J. 2018;39(20):1818–31. https://doi.org/10.1093/eurheartj/ehy085.
•• Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017;21(4):1009–20. https://doi.org/10.1016/j.celrep.2017.10.002 This paper describes a mechanism of antigen presentation by dendritic cells (DC) to T cells in salt-induced hypertension in mice. The authors demonstrated the role of salt that stimulates an amiloride-sensitive channel expressed on the surface of DCs, leading to enhanced activity of NADPH, increased ROS and generation of immunogenic isolevuglandins that form adducts with proteins and could be mediators of the interplay between innate and adaptive immunity in the pathophysiology of salt-sensitive hypertension.
Hevia D, Araos P, Prado C, Fuentes Luppichini E, Rojas M, Alzamora R, et al. Myeloid CD11c(+) antigen-presenting cells ablation prevents hypertension in response to angiotensin II plus high-salt diet. Hypertension. 2018;71(4):709–18. https://doi.org/10.1161/HYPERTENSIONAHA.117.10145.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. https://doi.org/10.1038/nri2506.
Shah KH, Shi P, Giani JF, Janjulia T, Bernstein EA, Li Y, et al. Myeloid suppressor cells accumulate and regulate blood pressure in hypertension. Circ Res. 2015;117(10):858–69. https://doi.org/10.1161/CIRCRESAHA.115.306539.
Juelke K, Romagnani C. Differentiation of human innate lymphoid cells (ILCs). Curr Opin Immunol. 2016;38:75–85. https://doi.org/10.1016/j.coi.2015.11.005.
Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, et al. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18(4):1093–102. https://doi.org/10.1681/ASN.2006070707.
Kossmann S, Schwenk M, Hausding M, Karbach SH, Schmidgen MI, Brandt M, et al. Angiotensin II-induced vascular dysfunction depends on interferon-gamma-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol. 2013;33(6):1313–9. https://doi.org/10.1161/ATVBAHA.113.301437.
Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16(11):1114–23. https://doi.org/10.1038/ni.3298.
Singh AK, Tripathi P, Cardell SL. Type II NKT cells: an elusive population with immunoregulatory properties. Front Immunol. 2018;9:1969. https://doi.org/10.3389/fimmu.2018.01969.
Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124(10):4642–56. https://doi.org/10.1172/JCI74084.
Chien YH, Meyer C, Bonneville M. gammadelta T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121–55. https://doi.org/10.1146/annurev-immunol-032713-120216.
Krebs CF, Lange S, Niemann G, Rosendahl A, Lehners A, Meyer-Schwesinger C, et al. Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin II-induced hypertension. Hypertension. 2014;63(3):565–71. https://doi.org/10.1161/HYPERTENSIONAHA.113.02620.
Paul S, Shilpi LG. Role of gamma-delta (gammadelta) T cells in autoimmunity. J Leukoc Biol. 2015;97(2):259–71. https://doi.org/10.1189/jlb.3RU0914-443R.
Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13(2):88–100. https://doi.org/10.1038/nri3384.
Saleh MA, Norlander AE, Madhur MS. Inhibition of interleukin 17-A but not interleukin-17F signaling lowers blood pressure and reduces end-organ inflammation in angiotensin II-induced hypertension. JACC Basic Transl Sci. 2016;1(7):606–16. https://doi.org/10.1016/j.jacbts.2016.07.009.
Li Y, Wu Y, Zhang C, Li P, Cui W, Hao J, et al. gammadelta T cell-derived interleukin-17A via an interleukin-1beta-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension. 2014;64(2):305–14. https://doi.org/10.1161/HYPERTENSIONAHA.113.02604.
Funding
The authors’ work was supported by the Canadian Institutes of Health Research (CIHR) grants 102606 and 123465, CIHR First Pilot Foundation Grant 143348, a Tier 1 Canada Research Chair (CRC) on Hypertension and Vascular Research by the CRC Government of Canada/CIHR Program, by the Canada Fund for Innovation (all to ELS), and by a fellowship to AC (Canadian Vascular Network).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Schiffrin reports grants from Canadian Institutes of Health Research and Servier France, personal fees from Novartis USA, and Servier Canada, outside the submitted work. The other authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Inflammation and Cardiovascular Diseases
Rights and permissions
About this article
Cite this article
Higaki, A., Caillon, A., Paradis, P. et al. Innate and Innate-Like Immune System in Hypertension and Vascular Injury. Curr Hypertens Rep 21, 4 (2019). https://doi.org/10.1007/s11906-019-0907-1
Published:
DOI: https://doi.org/10.1007/s11906-019-0907-1