Abstract
Purpose of Review
The aims of this meta-analysis were to investigate the effects of orally administered isolated taurine on resting systolic blood pressure (SBP) and diastolic blood pressure (DBP) in humans.
Recent Findings
There is growing evidence that taurine deficiency is associated with hypertension and that oral supplementation can have antihypertensive effects in humans. However, these investigations have been conducted across a number of decades and populations and have not been collectively reviewed. A search was performed using various databases in May 2018 and later screened using search criteria for eligibility. There were seven peer-reviewed studies meeting the inclusion criteria, encompassing 103 participants of varying age and health statuses. Taurine ingestion reduced SBP (Hedges’ g = − 0.70, 95% CI − 0.98 to − 0.41, P < 0.0001) and DBP (Hedges’ g = − 0.62, 95% CI − 0.91 to − 0.34, P < 0.0001). These results translated to mean ~ 3 mmHg reductions in both SBP (range = 0–15 mmHg) and DBP (range = 0–7 mmHg) following a range of doses (1 to 6 g/day) and supplementation periods (1 day to 12 weeks), with no adverse events reported.
Summary
These preliminary findings suggest that ingestion of taurine at the stated doses and supplementation periods can reduce blood pressure to a clinically relevant magnitude, without any adverse side effects. Future studies are needed to establish the effects of oral taurine supplementation on targeted pathologies and the optimal supplementation doses and periods.
Similar content being viewed by others
Introduction
Hypertension adversely affects public health worldwide and is a leading cause of cardiovascular and kidney disease [1]. As a major attributable cause of morbidity and mortality, hypertension significantly adds to the global disease burden [2]. Nutritional and lifestyle interventions are recognised as primary strategies for the prevention of hypertension and can reduce subsequent cardiovascular risk [3, 4]. Non-pharmacological management of hypertension is typically preferred for blood pressure control [5] to avoid the cost and potential side effects associated with chronic drug therapy [6]. However, pharmacological intervention is often necessary to elicit the desired control of blood pressure [7] but can be administered alongside secondary control measures to achieve the desired clinical outcomes [8].
Oral taurine can be supplemented through dietary sources or in isolated form and has been considered as a potential antihypertensive intervention [9]. Taurine is a sulfonic acid, found in both skeletal and cardiac myocytes in high concentrations [10]. While taurine can be synthesised from methionine and cysteine in mammals, it is also derived from food sources [11]. As a free, non-proteinogenic acid, it has capacity to take part in many intra- and extra-cellular biological processes—some of which have profound effects on organ-level physiology [12]. For example, taurine has antiarrhythmic, chronotropic and inotropic effects and has been suggested to reduce blood pressure [13]. Furthermore, the taurine transporter (TauT) is abundantly expressed in vascular smooth muscle [14], which supports its putative role as a vaso-relaxant in the control of vascular function [15]. The antihypertensive effects of taurine are attributed to its sympatholytic action on the central nervous system [16] and suppression of angiotensin II, which is a known stimulus of water retention in the development of cardiac preload [17]. The combination of these actions is thought to facilitate the hypotensive actions of taurine but studies are frequently limited to either in-vitro cell preparations or animal investigations and are seldom explored in humans. As such, it is necessary to evaluate the published evidence to establish an understanding of taurine’s effect on blood pressure in humans.
A WHO-coordinated Cardiac Diseases and Alimentary Comparison (CARDIAC) study, conducted across 60 separate populations from 22 countries, has reported inverse relationships between 24-h urinary taurine excretions and blood pressure, indicating a link between taurine deficiency and hypertension [18••]. Indeed, the popularity of taurine supplementation as an alternative therapy for mild-to-severely hypertensive patients has become increasingly apparent in recent years [9, 10, 17]. It is important to recognise the effects of oral taurine supplementation among humans, since the transfer of findings from rodent or in vitro studies remains speculative. Thus, it is necessary to understand whether tolerable doses of orally administered taurine can reduce blood pressure and confer cardio-protective effects. Therefore, this study systematically reviewed and meta-analysed all peer-reviewed studies that have provided human subjects with isolated taurine, with the intention of reducing resting blood pressure. Specifically, the aims of this meta-analysis were to investigate the effects of orally administered isolated taurine on resting SBP and DBP in humans.
Methods
Search Strategy
All literature investigating the effects of oral taurine on blood pressure was searched and obtained using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines, with a pre-determined search strategy [19]. There was no limit on the status or language of the publication and the final searches were performed in PubMed and Science Direct on 30.05.2018. The search terms independently used were ‘taurine AND blood pressure’, ‘taurine AND hypertension’ or ‘taurine AND cardiovascular’.
Study Selection
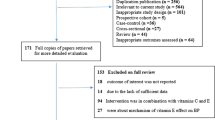
Following the initial identification of articles, two reviewers (MW and OJ) screened the titles and abstracts for inclusion, removing any duplicates. The reference lists of the initial papers were reviewed independently by two authors (MW and OJ). The remaining articles were then assessed by MW and OJ against the initial search criteria. To be included in this analysis, the studies must have (i) administered oral taurine to humans of any age or health status, without co-ingestion with other dietary supplements; (ii) evaluated resting systolic and diastolic blood pressure; and (iii) be placebo-controlled. Of the remaining papers, some were further removed for the reasons outlined in Fig. 1. In part, this included papers that had co-ingested taurine with other supplements, such as caffeine.
Data Extraction and Quality Assessment
Data were extracted independently by two authors (MW and OJ) and entered into a custom excel spreadsheet. Collected data included the following: (i) characteristics of the sample (sex, health status, age, activity status); (ii) study design; (iii) taurine dose; (iv) duration of supplementation; (v) post-intervention outcomes of systolic and diastolic blood pressure; and (vi) bias. Risk of bias was assessed by two authors (MW and OJ) according to Cochrane collaboration guidelines [20]. Publication bias was also accounted for by plotting the effect size as a percentage against the standard error (SE) for each study.
Statistical Analysis
Data analyses were performed by one author (MW). Raw data were extracted in the form of a mean, SD and sample size for the meta-analysis. Publicly available software (WebPlotDigitizer, Version 3.12) was used to extrapolate any unreported values from figures to raw mean and SD data. The overall meta-analysis of endurance performance was performed in RevMan 5.3, Cochrane Collaboration, Oxford, UK, including all seven of the final studies. The effect sizes and inverse-variance weights were calculated according to published equations Lipsey and Wilson [21]. Data were analysed with a fixed-effects model, with heterogeneity assessed using the I2 statistic. Hedges’ g and 95% confidence intervals were used to express the standardised mean differences between taurine and placebo groups across studies. The magnitudes of the effects were assessed based on the thresholds of 0.2, 0.5 and 0.8 for small, moderate and large, respectively [22]. Statistical significance was set at P < 0.05 for all analyses.
Results
Study Selection
The initial searches retrieved 7568 articles. After screening articles to remove reviews, duplicates and animal studies, 57 full-text articles remained. Searches of their reference lists did not reveal any missing papers. On the basis of the inclusion criteria, 50 articles were removed from the 57. Therefore, seven papers were included in the final meta-analysis (Fig. 1). Three of the removed papers met all of the inclusion criteria except for one, such as the use of placebo control [23] or not reporting both systolic and diastolic pressure [24].
Study Characteristics
The characteristics of the seven included studies are summarised in Table 1. The studies included a total of 103 participants, comprising both males and females of varying age, training and health status. Four of the studies recruited participants > 50 years, while four investigated patients with an ongoing clinical condition (i.e. type I diabetes, liver cirrhosis or heart disease). Five of the studies were independent groups designs, whilst two were cross-over designs. The range of taurine that was orally administered across studies was 1 to 6 g/day, with four studies providing ~ 1.5 g daily. Only one study provided an acute (single dose), while six studies gave chronic (multiple doses > 1 day), with 12 weeks being the longest supplementation period. There were no adverse events noted in any of the studies, beyond that reported in the control groups.
Meta-Analysis
The results of the overall meta-analyses for SBP and DBP are reported in Figs. 2 and 3, respectively. Overall, there was a ‘moderate’ improvement in systolic blood pressure with taurine compared to placebo (Hedges’ g = − 0.70, 95% CI = − 0.98 to − 0.41, P < 0.0001). The I2 statistic demonstrated 0% heterogeneity (P = 0.620). The meta-analysis for diastolic blood pressure also showed a moderate effect of taurine (Hedges’ g = − 0.62, 95% CI = − 0.91 to − 0.34, P < 0.0001), with 0% heterogeneity (I2, P = 0.500).
Effect of taurine on resting systolic blood pressure. Green circles = low risk of bias; red circles = high risk of bias; blank spaces = unclear risk of bias. A = random sequence generation; B = allocation concealment; C = blinding of participants and personnel; D = blinding of outcome assessment; E = incomplete outcome of data; F = selective reporting bias; G = other bias
Effect of taurine on resting diastolic blood pressure. Green circles = low risk of bias; red circles = high risk of bias; blank spaces = unclear risk of bias. A = random sequence generation; B = allocation concealment; C = blinding of participants and personnel; D = blinding of outcome assessment; E = incomplete outcome of data; F = selective reporting bias; G = other bias
Risk of Bias
The studies included had a generally low or unclear risk of bias. Most studies indicated a random allocation procedure but failed to provide the exact randomisation process or the way in which allocation to groups was concealed. Figures 4 and 5 show that publication bias analysis (standard mean differences and standard error relationship) was symmetrical, with no outliers for SBP and DBP, respectively.
Discussion
The main finding of this meta-analysis was that taurine significantly reduced both resting systolic and diastolic blood pressure by a ‘moderate’ magnitude. In absolute terms, the mean change in blood pressure across the studies was ~ 3 mmHg for both systolic and diastolic measurements, ranging between 0–15 and 0–7 mmHg, respectively. A change of this magnitude is considered to be clinically meaningful [7] and could significantly lower the risks of cardiovascular (CVD) and kidney disease. Indeed, reductions in SBP and DBP of > 2 mmHg can reduce the incidence of CVD by ~ 6% in both hypertensive and normotensive individuals [5]. The effect sizes noted herein are similar to those reported for a range of other dietary supplements, such as beetroot juice [32] (systolic ~ 4 mmHg, diastolic ~ 1 mmHg) or many classes of pharmacological interventions, such as angiotensin II receptor blockers [33] (systolic ~ 2 mmHg, diastolic ~ 0.2 mmHg), diuretics [34] (systolic ~ 6 mmHg, diastolic BP ~ 2 mmHg) and angiotensin-converting enzyme (ACE) inhibitors [35] (systolic ~ 5 mmHg, diastolic ~ 3 mmHg). Furthermore, unlike some of the above examples, there were no reported adverse side effects of taurine ingestion, despite the range of doses (1.5–6 g/day) and length of supplementation periods (1 day–12 weeks). Collectively, these preliminary findings suggest that ingestion of taurine across the above ranges and supplementation periods, which are markedly below the 10 g/day upper tolerable limit [36], can reduce blood pressure by a clinically-relevant magnitude.
The roles of taurine in the control of blood pressure are more comprehensively understood in the animal, rather than human model, yet some of the ascribed mechanisms could be feasibly shared. For example, the vaso-relaxant properties of taurine have been demonstrated in rodents [37,38,39], porcine [40] and the leporidae family [41], which has been more recently revealed in isolated human aortic rings [15]. The collective reasoning provided across the literature attributes these findings to taurine’s direct actions on potassium channels, situated in the smooth muscles or endothelial cells of the vasculature. Likewise, lower urinary norepinephrine excretion has been observed following taurine administration in humans, thus indicating the suppression of the sympathetic nervous system [24]. This supports the frequently reported reductions in sympathetic tone or attenuated over-activity of the sympathetic nervous system following taurine administration in animal studies [27, 42, 43]. One study included in the current meta-analysis [30•], which has provided some of the most convincing data to support the antihypertensive effects of taurine supplementation to date, reported an increased plasma hydrogen sulphide (H2S) level, which correlated with a reduction in blood pressure. The vasodilatory role of H2S has been previously described by Tian and colleagues [44] via its activation of ATP-sensitive potassium channels in rat coronary arteries and would explain the observed effects on blood pressure. Interestingly, perhaps the most commonly reported hypotensive mechanism of taurine in animal models is angiotensin II antagonism [45], which mimics the action of some prescribed drug therapies; however, to the best of the authors’ knowledge, this has not been investigated among humans.
The current literature describes the abundance of free taurine in human tissues and its involvement in the normal functioning of major physiological systems. Whilst many of these potential mechanisms could help to reduce blood pressure, it remains unclear whether the efficacy of taurine supplementation is reliant on the health status or age of the participants, which might influence the taurine content of various bodily tissues, such as that of the cardiovascular system. Unfortunately, the diverse characteristics of the meta-analysed participants included here do not facilitate the direct investigation of these questions. Nevertheless, the pre-established relationships between taurine deficiency and age [46], heart disease [47], hypertension [18••] and diabetes [48] indicate that features of these conditions are partly explained by a pre-existing taurine deficiency, as indicated by higher 24-h urinary taurine excretion rates. In the resting state, young healthy participants are likely to have a sufficient content of taurine stored in muscle tissue [12, 49], which would infer a redundancy of taurine supplementation among these participants. However, there are two studies included in the current meta-analysis with young (< 30 years), normotensive participants [28, 31], where contrasting results are reported. For example, Moloney and colleagues [31] investigated 2 weeks of taurine supplementation among type I diabetic patients and found no change in blood pressure, while Warnock et al. [28] provided a single acute dose of taurine and reported a mean reduction of 7 mmHg systolic pressure. An apparent differentiating factor between these two studies was the baseline blood pressure values, which were surprisingly low (~ 100/86 mmHg) among the young diabetic cohort [31], yet borderline hypertensive (~ 127/73 mmHg) among the young healthy cohort [28]. Furthermore, despite no change in blood pressure, taurine supplementation reversed endothelial dysfunction and arterial stiffness in conduit vessels of the diabetic group [31], indicating that taurine supplementation targeted pathological changes in the cardiovascular system, without altering arterial pressure. Therefore, it is possible that the effects of taurine supplementation on blood pressure are pronounced among pre-hypertensive or hypertensive participants and do not affect those with normal or moderately low blood pressure, irrespective of their health status. Further research is clearly required to extend the preliminary summary of studies provided here to establish the populations that are most likely to benefit from taurine supplementation.
It was apparent that most studies selected to provide repeated, long-term doses of taurine (> 1 day; Table 1), rather than acute administration (≤ 1 day). The reasons for this are unclear, but two recent meta-analyses have identified that acute taurine supplementation was sufficient to improve endurance exercise performance [50, 51], which among other factors, depends upon the capacity and efficiency of the cardiovascular system. Indeed, some studies have showed a concomitant reduction in heart rate following acute taurine supplementation [28, 52] and taurine-containing energy drinks are known to increase stroke volume in humans [53]. Furthermore, taurine has a short half-life and 1 week of supplementation does not alter skeletal muscle content in healthy participants [54]. Given these short-term effects in apparently healthy participants, the rationale for repeated ingestion is not entirely clear but would presumably target taurine deficient patients only, based on the theory that continuous ingestion will augment endogenous storage. There was an insufficient amount of articles available to perform a sub-analysis of acute and chronic supplementation regimes or populations, highlighting the need for further research in this area. Evidence-based guidelines for the optimal supplementation periods and dosages of oral taurine for the purpose of cardio-protection are yet to be determined, yet are necessary to develop the applicability of fundamental research conducted across the last ~ 30 years. Indeed, a long-term diet rich in taurine might offset hypertension to a similar magnitude, yet this has not been empirically explored.
The risk of bias across the studies included in this meta-analysis was generally low, with minor exceptions for ‘unclear’ risks of random allocation procedures and allocation concealment (Figs. 2 and 3). However, there were other papers not included in the meta-analysis for basic scientific reasons, such as lack of control groups (i.e. [23]) or incomplete reporting of outcome variables [24]. This is unfortunate, as the addition of these papers might have strengthened the overall effects of taurine on blood pressure; however, this highlights the need for more detailed and rigorous research design in this area. In addition, it is possible that certain populations have been underrepresented across the available meta-analysed studies. The studies were conducted in Europe [28, 29, 31], Asia [27, 30] and the Middle East [25, 26], yet there were no studies conducted in larger continents, such as the North and South America, Africa or Australasia, where similar risks of mortality due to cardiovascular disease are present and increased in certain ethnic groups [55]. This could be partly addressed with changes in dietary supplementation, including changes in taurine intake. Indeed, there are markedly different dietary habits between populations of the same country and taurine deficiency, as a result of an unintentionally low taurine diet, has been associated with hypertension [56]. A larger number of studies are needed in these underrepresented geographical areas to ascertain the validity of these association studies on a global scale.
Conclusions
Given the small number of studies meta-analysed, our data should be considered as a preliminary assessment of the antihypertensive effects of oral taurine supplementation. Nevertheless, a mean ~ 3 mmHg (range 0–15 mmHg) change in SBP across 103 participants represents a substantial contribution towards the 5 mmHg reductions in SBP that were estimated to decrease the risk of mortality due to stroke by 14% and mortality from cardiovascular diseases by 9% [7]. Furthermore, it is noteworthy that there have been no adverse events associated with taurine ingestion, indicating its tolerability among human participants. The studies included in this meta-analysis included a range of subjects, from young healthy and recreationally active, to elderly and diseased, thus confirming the wider application of taurine supplementation but restricting the understanding of taurine’s therapeutic role in target pathologies. Research is required to establish the correct taurine doses and supplementation periods required for the management of cardiovascular disease or associated risk factors. Further studies investigating dietary sources of taurine and their influence on risk factors of cardiac disease are required to provide more comprehensive evidence of taurine’s health benefits.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major Importance
WHO Raised blood pressure: situation and trends. Global Health Observatory. http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/ (accessed 04 May 2018).
Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJL. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60.
Smith SC, Benjamin EJ, Bonow RO, et al. World Heart Federation and the Preventive Cardiovascular Nurses Association. Circulation. 2011;124:2458–73.
Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC VII report. JAMA. 2003;289:2560–72.
Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–9.
Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308.
COLLABORATION BPLTT. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35.
Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;30:365–401.
Xu Y, Arneja AS, Tappia PS, Dhalla NS. The potential health benefits of taurine in cardiovascular disease. Exp Clin Cardiol. 2008;13:57–65.
Schaffer SW, Ito T, Azuma J. Clinical significance of taurine. Amino Acids. 2014;46:1–5.
Laidlaw SA, Grosvenor M, Kopple JD. The taurine content of common foodstuffs. Parenter Enter Nutr. 1990;14:183–8.
Huxtable JR. Physiological actions of taurine. Physiol Rev. 1992;72:101–63.
Satoh H, Sperelakis N. Review of some actions of taurine on ion channels of cardiac muscle cells and others. Gen Pharmacol. 1998;30:451–63.
Liao XB, Zhou XM, Li JM, Tan ZP, Liu LM, Zhang W, et al. Taurine transporter is expressed in vascular smooth muscle cells. Amino Acids. 2007;33:639–43.
Ulusoy KG, Kaya E, Karabacak K, Seyrek M, Duvan İ, Yildirim V, et al. Taurine relaxes human radial artery through potassium channel opening action. Korean J Physiol Pharmacol. 2017;21:617–23.
Yoshioka M, Takasugi Y, Koga Y. Central hypotensive effect involving neurotransmitters of long-term administration of taurine to stroke-prone spontaneously hypertensive rat. Masui. 2007;56:139–47.
Ito T, Schaffer S, Azuma J. The effect of taurine on chronic heart failure: actions of taurine against catecholamine and angiotensin II. Amino Acids. 2014;46:111–9.
•• Sagara M, Murakami S, Mizushima S, et al. Taurine in 24-h urine samples is inversely related to cardiovascular risks of middle aged subjects in 50 populations of the world. Adv Exp Med Biol. 2015;803:623–36. This multi-centre, cross-sectional study of > 4,000 patients demonstrated an inverse relationship between 24-h urinary taurine/creatinine ratios and both hypertension and obesity, highlighting the links between taurine deficiency and cardiovascular disease risk.
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions: cochrane book series. Chichester: Wiley; 2008.
Lipsey MW, Wilson DB. Practical meta-analysis. London: Sage; 2001.
Rosenthal R, Rosnow RL. Essentials of behavioral research: methods and data analysis. New York: McGraw-Hill; 1984.
Satoh H, Kang J. Modulation by taurine of human arterial stiffness and wave reflection. Adv Exp Med Biol. 2009;643:47–55.
Mizushima S, Nara Y, Sawamura M, et al. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol. 1996;403:615–22.
Ahmadian M, Roshan VD, Ashourpore E. Taurine supplementation improves functional capacity, myocardial oxygen consumption, and electrical activity in heart failure. J Diet Suppl. 2017;14:422–32.
Beyranvand MR, Khalafi MK, Roshan VD, Choobineh S, Parsa SA, Piranfar MA. Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol. 2011;57:333–7.
Fujita T, Ando K, Noda H, Ito Y, Sato Y. Effects of increased adrenomedullary activity and taurine in young patients with borderline hypertension. Circulation. 1987;75:525–32.
Warnock R, Jeffries O, Patterson S, Waldron M. The effects of caffeine, taurine or caffeine-taurine co-ingestion on repeat-sprint cycling performance and physiological responses. Int J Sports Physiol Perform. 2017;24:1–24.
Schwarzer R, Kivaranovic D, Mandorfer M. Randomised clinical study: the effects of oral taurine 6g/day vs placebo on portal hypertension. Aliment Pharmacol Ther. 2018;47:86–94.
• Sun Q, Wang B, Li Y, et al. Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: randomized, double-blind, placebo-controlled study. Hypertension. 2016;67:541–9. This randomized, double-blind, placebo-controlled study demonstrated that oral taurine supplementation lowered blood pressure in a large cohort ( n = 120) of pre-hypertensive participants and provided the first mechanistic understanding of taurine’s effects on the vasculature.
Moloney MA, Casey RG, O'Donnell DH, Fitzgerald P, Thompson C, Bouchier-Hayes DJ. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diab Vasc Dis Res. 2010;7:300–10.
Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr. 2013;143:818–26.
Zhao D, Liu H, Dong P. A meta-analysis of antihypertensive effect of telmisartan versus candesartan in patients with essential hypertension. Clin Exp Hypertens. 2018;28:1–5.
Liang W, Ma H, Cao L, Yan W, Yang J. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med. 2017;21:2634–42.
Peck RN, Smart LR, Beier R, Liwa AC, Grosskurth H, Fitzgerald DW, et al. Difference in blood pressure response to ACE-inhibitor monotherapy between black and white adults with arterial hypertension: a meta-analysis of 13 clinical trials. BMC Nephrol. 2013;14:201.
Shao A, Hathcock JN. Risk assessment for the amino acids taurine, L-glutamine and Larginine. Regul Toxicol Pharmacol. 2008;50:376–99.
Ristori MT, Verdetti J. Effects of taurine on rat aorta in vitro. Fundam Clin Pharmacol. 1991;5:245–58.
Niu LG, Zhang MS, Liu Y, Xue WX, Liu DB, Zhang J, et al. Vasorelaxant effect of taurine is diminished by tetraethylammonium in rat isolated arteries. Eur J Pharmacol. 2008;580:169–74.
Nishida S, Satoh H. Vascular modulation of rat aorta by taurine. Adv Exp Med Biol. 2009;643:37–46.
Liu Y, Niu L, Zhang W, Cui L, Zhang X, Liang Y, et al. Effect of taurine on contractions of the porcine coronary artery. Pharmacol Rep. 2009;61:681–9.
Fanconi F, Giotti A, Manzini S, et al. The effect of taurine on high potassium- and noradrenaline-induced contraction in rabbit ear artery. Br J Pharmacol. 1982;75:605–12.
Fujita T, Sato Y. The antihypertensive effect of taurine in DOCA-salt rats. J Hypertens Suppl. 1984;2:S563–5.
Hano T, Kasano M, Tomari H, et al. Taurine suppresses pressor response through the inhibition of sympathetic nerve activity and the improvement in baro-reflex sensitivity of spontaneously hypertensive rats. Adv Exp Med Biol. 2009;643:57–63.
Tian XY, Wong WT, Sayed N, Luo J, Tsang SY, Bian ZX, et al. NaHS relaxes rat cerebral artery in vitro via inhibition of l-type voltage-sensitive Ca2+ channel. Pharmacol Res. 2012;65:239–46.
Li C, Cao L, Zeng Q, Liu X, Zhang Y, Dai T, et al. Taurine may prevent diabetic rats from developing cardiomyopathy also by downregulating angiotensin II type 2 receptor expression. Cardiovasc Drugs Ther. 2005;19:105–12.
Jeevanandam M, Young DH, Ramias L, Schiller WR. Effect of major trauma on plasma free amino acid concentrations in geriatric patients. Am J Clin Nutr. 1990;51:1040–5.
Sole MJ, Jeejeebhoy KN. Conditioned nutritional requirements and the pathogenesis and treatment of myocardial failure. Curr Opin Clin Nutr Metab Care. 2000;3:417–24.
Franconi F, Bennardini F, Mattana A, Miceli M, Ciuti M, Mian M, et al. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: effects of taurine supplementation. Am J Clin Nutr. 1995;61:1115–9.
Henriksson J. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. J Exp Biol. 1991;160:149–65.
Souza DB, Del Coso J, Casonatto J, et al. Acute effects of caffeine-containing energy drinks on physical performance: a systematic review and meta-analysis. Eur J Nutr. 2017;56:13–27.
Waldron M, Patterson SD, Tallent J, Jeffries O. The effects of an oral taurine dose and supplementation period on endurance exercise performance in humans: a meta-analysis. Sports Med. 2018;48:1247–53.
Waldron M, Knight F, Tallent J, Patterson S, Jeffries O. The effects of taurine on repeat sprint cycling after low or high cadence exhaustive exercise in females. Amino Acids. 2018;50:663–9.
Baum M, Weiss M. The influence of a taurine containing drink on cardiac parameters before and after exercise measured by echocardiography. Amino Acids. 2001;20:75–82.
Galloway SD, Talanian JL, Shoveller AK, et al. Seven days of oral taurine supplementation does not increase muscle taurine content or alter substrate metabolism during prolonged exercise in humans. J Appl Physiol. 2008;105:643–51.
Ong KL, Cheung BMY, Man YB, Lau CP, Lam KSL. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75.
Nara Y, Zhao GS, Huang ZD, et al. Relationship between dietary factors and blood pressure in China. J Cardiovasc Pharmacol. 1990;16(Suppl. 8):S40–2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Antihypertensive Agents: Mechanisms of Drug Action
Rights and permissions
About this article
Cite this article
Waldron, M., Patterson, S.D., Tallent, J. et al. The Effects of Oral Taurine on Resting Blood Pressure in Humans: a Meta-Analysis. Curr Hypertens Rep 20, 81 (2018). https://doi.org/10.1007/s11906-018-0881-z
Published:
DOI: https://doi.org/10.1007/s11906-018-0881-z