Abstract
Purpose of Review
The goal of this review is to present the newest insights into what we view as a central failure of cardiovascular adaptation in preeclampsia (PE) by focusing on one clinically significant manifestation of maternal endothelial dysfunction: nitric oxide signaling. The etiology, symptoms, and current theories of the PE syndrome are described first, followed by a review of the available evidence, and underlying causes of reduced endothelial nitric oxide (NO) signaling in PE.
Recent Findings
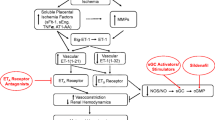
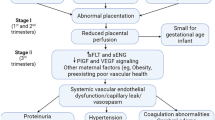
PE maladaptations include, but are not limited to, altered physiological stimulatory inputs (e.g., estrogen; VEGF/PlGF; shear stress) and substrates (L-Arg; ADMA), augmented placental secretion of anti-angiogenic and inflammatory factors such as sFlt-1 and Eng, changes in eNOS (polymorphisms, expression), and reduced bioavailability of NO secondary to oxidative stress.
Summary
PE is a complex obstetrical syndrome that is associated with maternal vascular dysfunction. Diminished peripheral endothelial vasodilator influence in general, and of NO signaling specifically, are key in driving disease progression and severity.

Similar content being viewed by others
Abbreviations
- 2-ME:
-
2-Methoxyestradiol
- ADMA:
-
Asymmetric dimethyl arginine
- AT1:
-
Angiotensin type 1 receptor
- AT2:
-
Angiotensin type 2 receptor
- BH4 :
-
Tetrahydrobiopterin
- CaM:
-
Calmodulin
- cGMP:
-
Cyclic guanosine 3′,5′ monophosphate
- DDAH:
-
Dimethylarginine dimethylaminohydrolase
- ECE:
-
Endothelin converting enzyme
- EDH:
-
Endothelium-derived hyperpolarizing factor
- EDRF:
-
Endothelium-derived relaxing factor
- ET:
-
Endothelin
- ETA :
-
Endothelin A receptor
- ETB :
-
Endothelin B receptor
- eNOS/NOS3:
-
Endothelial nitric oxide synthase
- ERα:
-
Estrogen receptor-α
- ERß:
-
Estrogen receptor-ß
- ERRϒ:
-
Estrogen-related receptor ϒ
- FAD:
-
Flavin adenine dinucleotide
- FMD:
-
Flow-mediated dilation
- FMN:
-
Flavin mononucleotide
- Gi :
-
Gi-coupled receptor
- GPER:
-
G-protein-coupled estrogen receptor
- HO-1:
-
Heme oxygenase-1
- iNOS/NOS2:
-
Cytokine-inducible nitric oxide synthase
- nNOS/NOS1:
-
Neuronal nitric oxide synthase
- L-Arg:
-
L-arginine
- LOX-1:
-
Lectin-like oxidized LDL receptor-1
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NO:
-
Nitric oxide
- oxLDL:
-
Oxidized low-density lipoproteins
- P2Y:
-
Purinergic G protein-coupled receptor
- PE:
-
Preeclampsia
- PlGF:
-
Placental growth factor
- PRMT:
-
S-adenylmethionine-dependent methyltransferase
- ROS:
-
Reactive oxygen species
- RUPP:
-
Reduced uterine perfusion pressure
- sEng:
-
Soluble endoglin
- sFlt-1:
-
Soluble fms-like tyrosine kinase-1 receptor
- SIRT-1:
-
Silent mating-type information regulation 2 homolog 1
- SOD:
-
Superoxide dismutases
- STBEV:
-
Syncytiotrophoblast extracellular vesicle
- TRPV1:
-
Transient receptor potential cation channel subfamily V member 1
- TRPV4:
-
Transient receptor potential cation channel subfamily V member 4
- VEGF:
-
Vascular endothelial growth factor
- VEGFR1:
-
VEGF receptor 1, Flt1
- VEGFR2:
-
VEGF receptor 2, KDR
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Osol G, Bernstein I. Preeclampsia and maternal cardiovascular disease: consequence or predisposition? J Vasc Res. 2014;51(4):290–304. A paper that considers whether long-term health consequences of PE on a woman’s cardiovascular health result from the disease, or if the disease results from a phenotype that already has some cardiovascular damage, so that the stress of pregnancy results in maladptation and leads to the symptoms of PE.
• Brosens I. A study of the spiral arteries of the decidua basalis in normotensive and hypertensive pregnancies. J Obstet Gynaecol Br Commonw. 1964;71:222–30. Initial observation of shallow spiral artery invasion in PE women more than 50 years ago.
Karumanchi SA. Angiogenic factors in preeclampsia: from diagnosis to therapy. Hypertension. 2016;67(6):1072–9.
Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–7.
Van Wijk MJ, et al. Vascular function in preeclampsia. Cardiovasc Res. 2000;47(1):38–48.
Roberts JM, Bell MJ. If we know so much about preeclampsia, why haven't we cured the disease? J Reprod Immunol. 2013;99(1–2):1–9.
Myatt L, Roberts JM. Preeclampsia: syndrome or disease? Curr Hypertens Rep. 2015;17(11):83.
Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 2013;62(6):1046–54.
Burke SD, et al. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension. 2010;55(3):729–37.
• Huppertz B, Weiss G, Moser G. Trophoblast invasion and oxygenation of the placenta: measurements versus presumptions. J Reprod Immunol. 2014;101-102:74–9. Challenges the theory that poor trophoblast invasion leads to placental hypoxia and invites the reader to rethink the hypothesis that has almost become dogma in terms of the pathogenesis of PE.
Bernstein IM, et al. Intolerance to volume expansion: a theorized mechanism for the development of preeclampsia. Obstet Gynecol. 1998;92(2):306–8.
Hutchinson ES, et al. Utero-placental haemodynamics in the pathogenesis of pre-eclampsia. Placenta. 2009;30(7):634–41.
Gonska BD, Bethge KP, Kreuzer H. Spontaneous and stimulus-induced arrhythmia behavior in dilated cardiomyopathy. Z Kardiol. 1987;76(9):546–53.
Nasiri R, et al. Association of meteorological factors and seasonality with preeclampsia: a 5-year study in northeast of Iran. Clin Exp Hypertens. 2014;36(8):586–9.
George EM. New approaches for managing preeclampsia: clues from clinical and basic research. Clin Ther. 2014;36(12):1873–81.
Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232(1):R27–44.
• Osol G, et al. Placental growth factor is a potent vasodilator of rat and human resistance arteries. Am J Physiol Heart Circ Physiol. 2008;294(3):H1381–7. Our study describing the vasoactive effects of PlGF (and, therefore, of the VEGFR1/Flt-1 endothelial receptor) in isolated vessels from humans and rats, showing that it is a potent vasodilator and that its action derives largely from stimulation NO production.
• Cross SN, et al. Bevacizumab-mediated interference with VEGF signaling is sufficient to induce a preeclampsia-like syndrome in nonpregnant women. Rev Obstet Gynecol. 2012;5(1):2–8. This paper is notable in its demonstration that a preeclampsia-like condition (hypertension, proteinuria) can be induced in nonpregnant women simply by taking an antibody that binds VEGF, much like sFlt-1.
Svedas E, et al. Vascular endothelial growth factor induced functional and morphologic signs of endothelial dysfunction in isolated arteries from normal pregnant women. Am J Obstet Gynecol. 2003;188(1):168–76.
Magness RR, et al. Endothelial vasodilator production by uterine and systemic arteries. V. Effects of ovariectomy, the ovarian cycle, and pregnancy on prostacyclin synthase expression. Prostaglandins Other Lipid Mediat, 2000. 60(4-6):103-18.
Sheibani L, et al. Augmented H2S production via cystathionine-beta-synthase upregulation plays a role in pregnancy-associated uterine vasodilation. Biol Reprod, 2017. 96(3): p. 664-672.
Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol, 2010. 299(5): p. H1642-52.
Gokina NI, Goecks T. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am J Physiol Heart Circ Physiol, 2006. 290(5):H2124-35.
Steinert JR, et al. Redox modulation of Ca2+ signaling in human endothelial and smooth muscle cells in pre-eclampsia. Antioxid Redox Signal, 2009. 11(5): p. 1149-63.
• Furchgott RF, Zawadzk JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature, 1980. 288(5789): p. 373-6. A classic paper, the first discovery that the endothelium exerts a vasodilatory influence on vascular smooth muscle in response to cholinergic stimulation.
Furchgott RF. The 1996 Albert Lasker medical research awards. The discovery of endothelium-derived relaxing factor and its importance in the identification of nitric oxide. JAMA. 1996;276(14):1186–8.
• Osol G, et al. Inhibition of nitric oxide synthases abrogates pregnancy-induced uterine vascular expansive remodeling. J Vasc Res. 2009;46(5):478–86. Description of how systemic eNOS inhibition with L-NAME attenuates expansive remodeling of uterine vessels in pregnant rats, implicating NO in maternal uterine vascular remodeling during pregnancy.
Hale SA, et al. Reduced NO signaling during pregnancy attenuates outward uterine artery remodeling by altering MMP expression and collagen and elastin deposition. Am J Physiol Heart Circ Physiol. 2011;301(4):H1266–75.
van der Heijden OW, et al. Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod. 2005;72(5):1161–8.
Crews JK, et al. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35(1 Pt 2):367–72.
Buhimschi I, et al. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol. 1995;172(5):1577–84.
Goncalves-Rizzi VH, et al. Sodium nitrite attenuates hypertension-in-pregnancy and blunts increases in soluble fms-like tyrosine kinase-1 and in vascular endothelial growth factor. Nitric Oxide. 2016;57:71–8.
Choi JW, Im MW, Pai SH. Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann Clin Lab Sci. 2002;32(3):257–63.
Seligman SP, et al. The role of nitric oxide in the pathogenesis of preeclampsia. Am J Obstet Gynecol. 1994;171(4):944–8.
Silver RK, et al. Evaluation of nitric oxide as a mediator of severe preeclampsia. Am J Obstet Gynecol. 1996;175(4 Pt 1):1013–7.
Pathak N, et al. Estimation of oxidative products of nitric oxide (nitrates, nitrites) in preeclampsia. Aust N Z J Obstet Gynaecol. 1999;39(4):484–7.
Pimentel AM, et al. L-arginine-nitric oxide pathway and oxidative stress in plasma and platelets of patients with pre-eclampsia. Hypertens Res. 2013;36(9):783–8.
Eleuterio NM, et al. Relationship between adiponectin and nitrite in healthy and preeclampsia pregnancies. Clin Chim Acta. 2013;423:112–5.
• Sandrim VC, et al. Nitric oxide formation is inversely related to serum levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 and soluble endogline in preeclampsia. Hypertension. 2008;52(2):402–7. Provides clinical evidence for impaired NO formation in PE or gestational hypertension, and makes a case for sFlt-1 and sEng inhibiting NO formation based on significant negative correlation between antiangiogenic factors and circulating nitrite concentrations. sThe r 2 values were on the order of 0.25 and 0.36 for sFlt-1 and sEng, respectively.
Zeng Y, et al. Homocysteine, endothelin-1 and nitric oxide in patients with hypertensive disorders complicating pregnancy. Int J Clin Exp Pathol. 2015;8(11):15275–9.
Pettersson A, Hedner T, Milsom I. Increased circulating concentrations of asymmetric dimethyl arginine (ADMA), an endogenous inhibitor of nitric oxide synthesis, in preeclampsia. Acta Obstet Gynecol Scand. 1998;77(8):808–13.
Sankaralingam S, Xu H, Davidge ST. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc Res. 2010;85(1):194–203.
Bernardi FC, et al. Plasma nitric oxide, endothelin-1, arginase and superoxide dismutase in the plasma and placentae from preeclamptic patients. An Acad Bras Cienc. 2015;87(2):713–9.
Rytlewski K, et al. Effects of prolonged oral supplementation with l-arginine on blood pressure and nitric oxide synthesis in preeclampsia. Eur J Clin Investig. 2005;35(1):32–7.
Camarena Pulido EE, et al. Efficacy of L-arginine for preventing preeclampsia in high-risk pregnancies: a double-blind, randomized, clinical trial. Hypertens Pregnancy. 2016;35(2):217–25.
Vadillo-Ortega F, et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342:d2901.
Khalil AA, et al. Asymmetric dimethylarginine, arginine and homoarginine at 11-13 weeks' gestation and preeclampsia: a case-control study. J Hum Hypertens. 2013;27(1):38–43.
Lopez-Alarcon M, et al. Serial determinations of asymmetric dimethylarginine and homocysteine during pregnancy to predict pre-eclampsia: a longitudinal study. BJOG. 2015;122(12):1586–92.
Zheng JJ, et al. Assessment of ADMA, estradiol, and progesterone in severe preeclampsia. Clin Exp Hypertens. 2016;38(4):347–51.
Laskowska M, Laskowska K, Oleszczuk J. PP135. Maternal serum levels of endothelial nitric oxide synthase and ADMA, an endogenous ENOS inhibitor in pregnancies complicated by severe preeclampsia. Pregnancy Hypertens. 2012;2(3):312.
Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24(6):1023–30.
Siroen MP, et al. The clinical significance of asymmetric dimethylarginine. Annu Rev Nutr. 2006;26:203–28.
Bian Z, Shixia C, Duan T. First-trimester maternal serum levels of sFLT1, PGF and ADMA predict preeclampsia. PLoS One. 2015;10(4):e0124684.
Gumus E, et al. Possible role of asymmetric dimethylarginine (ADMA) in prediction of perinatal outcome in preeclampsia and fetal growth retardation related to preeclampsia. J Matern Fetal Neonatal Med. 2016;29(23):3806–11.
Alpoim PN, et al. Assessment of L-arginine asymmetric 1 dimethyl (ADMA) in early-onset and late-onset (severe) preeclampsia. Nitric Oxide. 2013;33:81–2.
Boger RH, et al. The role of nitric oxide synthase inhibition by asymmetric dimethylarginine in the pathophysiology of preeclampsia. Gynecol Obstet Investig. 2010;69(1):1–13.
Anderssohn M, et al. Severely decreased activity of placental dimethylarginine dimethylaminohydrolase in pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2012;161(2):152–6.
Ehsanipoor RM, et al. Nitric oxide and carbon monoxide production and metabolism in preeclampsia. Reprod Sci. 2013;20(5):542–8.
Akbar F, et al. Haplotypic association of DDAH1 with susceptibility to pre-eclampsia. Mol Hum Reprod. 2005;11(1):73–7.
Kromer W, et al. Direct comparison between the ulcer-healing effects of two H(+)-K(+)-ATPase inhibitors, one M1-selective antimuscarinic and one H2 receptor antagonist in the rat. Pharmacology. 1990;41(6):333–7.
Maas R. Pharmacotherapies and their influence on asymmetric dimethylargine (ADMA). Vasc Med. 2005;10(Suppl 1):S49–57.
• Vanhoutte PM, et al. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res. 2016;119(2):375–96. A detailed, illustrated, tour-de-force review by one of the leading investigators in the field that covers NO signaling, mostly in normal rather than pathological conditions. Well-illustrated and comprehensive.
Duckles SP, Miller VM. Hormonal modulation of endothelial NO production. Pflugers Arch. 2010;459(6):841–51.
Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60(2):210–41.
Meyer MR, et al. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308(1–2):9–16.
Haas E, et al. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104(3):288–91.
Tropea T, et al. Pregnancy augments G protein estrogen receptor (GPER) induced Vasodilation in rat uterine arteries via the nitric oxide - cGMP signaling pathway. PLoS One. 2015;10(11):e0141997.
Lee DK, Nevo O. 2-Methoxyestradiol regulates VEGFR-2 and sFlt-1 expression in human placenta. Placenta. 2015;36(2):125–30.
Shen Z, et al. Decreased maternal serum 2-methoxyestradiol levels are associated with the development of preeclampsia. Cell Physiol Biochem. 2014;34(6):2189–99.
Berkane N, et al. From pregnancy to preeclampsia: a key role for estrogens. Endocr Rev. 2017;38(2):123–44.
Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension. 2013;61(2):480–7.
Fernandez AR, Omar SZ, Husain R. Role of Genistein in preeclampsia: a case-control study. J Reprod Med. 2016;61(1–2):47–51.
Ni Y, et al. Pregnancy augments uteroplacental vascular endothelial growth factor gene expression and vasodilator effects. Am J Phys. 1997;273(2 Pt 2):H938–44.
Ni Y, Meyer M, Osol G. Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. Am J Obstet Gynecol. 1997;176(4):856–64.
Boeldt DS, et al. Positive versus negative effects of VEGF165 on Ca2+ signaling and NO production in human endothelial cells. Am J Physiol Heart Circ Physiol. 2017;312(1):H173–81.
Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95(9):884–91.
• Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. A study that shored up the case for the importance of sFlt-1 in PE, and led to an interesting essay in the New Yorker (The Preeclampsia Puzzle) that described the medical, scientific and human background of this discovery. Available for free on the web ( http://www.newyorker.com/magazine/2006/07/24/the- preeclampsia-puzzle).
Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78.
Mangos GJ, et al. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens. 2012;30(2):351–8.
Lommerse T, et al. Endothelium-dependent vasodilatation in formerly preeclamptic women correlates inversely with body mass index and varies independently of plasma volume. Reprod Sci. 2007;14(8):765–70.
Germain AM, et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49(1):90–5.
Chambers JC, et al. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285(12):1607–12.
Hamad RR, et al. Impaired endothelial function and elevated levels of pentraxin 3 in early-onset preeclampsia. Acta Obstet Gynecol Scand. 2012;91(1):50–6.
Paez O, et al. Parallel decrease in arterial distensibility and in endothelium-dependent dilatation in young women with a history of pre-eclampsia. Clin Exp Hypertens. 2009;31(7):544–52.
Goynumer G, et al. Vascular risk in women with a history of severe preeclampsia. J Clin Ultrasound. 2013;41(3):145–50.
Yinon Y, et al. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction: insights into future vascular risk. Circulation. 2010;122(18):1846–53.
Weissgerber TL, et al. Impaired flow-mediated dilation before, during, and after preeclampsia: a systematic review and meta-analysis. Hypertension. 2016;67(2):415–23.
• Kublickiene KR, et al. Preeclampsia: evidence for impaired shear stress-mediated nitric oxide release in uterine circulation. Am J Obstet Gynecol. 2000;183(1):160–6. A study on isolated subcutaneous arteries from women who had a normal vs. PE pregnancy that identifies loss of shear stress-induced NO vasodilation. This illustrates a selective ‘lesion’ in PE (since acetylcholine-induced endothelial responses were normal) and the fact that it is still present several years after a PE pregnancy.
Nelson SH, et al. Pregnancy augments nitric oxide-dependent dilator response to acetylcholine in the human uterine artery. Hum Reprod. 1998;13(5):1361–7.
Nelson SH, et al. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circ Res. 2000;87(5):406–11.
Morschauser TJ, et al. Local effects of pregnancy on connexin proteins that mediate Ca2+- associated uterine endothelial NO synthesis. Hypertension. 2014;63(3):589–94.
Joyce JM, et al. Endothelial vasodilator production by uterine and systemic arteries. IX. eNOS gradients in cycling and pregnant ewes. Am J Physiol Heart Circ Physiol. 2002;282(1):H342–8.
Laskowska M, Laskowska K, Oleszczuk J. The relation of maternal serum eNOS, NOSTRIN and ADMA levels with aetiopathogenesis of preeclampsia and/or intrauterine fetal growth restriction. J Matern Fetal Neonatal Med. 2015;28(1):26–32.
Laskowska M, et al. A comparison of maternal serum levels of endothelial nitric oxide synthase, asymmetric dimethylarginine, and homocysteine in normal and preeclamptic pregnancies. Med Sci Monit. 2013;19:430–7.
Aleman I, et al. Endothelial and inducible nitric oxide synthase expression in Venezuelan patients with pre-eclampsia. Investig Clin. 2008;49(3):321–30.
Mazzanti L, et al. Nitric oxide and peroxynitrite platelet levels in gestational hypertension and preeclampsia. Platelets. 2012;23(1):26–35.
Ramadoss J, Pastore MB, Magness RR. Endothelial caveolar subcellular domain regulation of endothelial nitric oxide synthase. Clin Exp Pharmacol Physiol. 2013;40(11):753–64.
Smith-Jackson K, et al. Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta. 2015;36(5):607–10.
Myatt L, et al. Endothelial nitric oxide synthase in placental villous tissue from normal, pre-eclamptic and intrauterine growth restricted pregnancies. Hum Reprod. 1997;12(1):167–72.
Kim YJ, et al. Reduced L-arginine level and decreased placental eNOS activity in preeclampsia. Placenta. 2006;27(4–5):438–44.
Orange SJ, et al. Placental endothelial nitric oxide synthase localization and expression in normal human pregnancy and pre-eclampsia. Clin Exp Pharmacol Physiol. 2003;30(5–6):376–81.
Motta-Mejia C, et al. Placental vesicles carry active endothelial nitric oxide Synthase and their activity is reduced in preeclampsia. Hypertension. 2017;70(2):372–81.
Maria Procopciuc L. et al., Maternal/fetal eNOS-Glu298Asp genotypes and their influence on the severity, prognosis, and lipid profile of preeclampsia. J Matern Fetal Neonatal Med 2017: p. 1–8.
Sakar MN, et al. Association of endothelial nitric oxide synthase gene G894T polymorphism and serum nitric oxide levels in patients with preeclampsia and gestational hypertension. J Matern Fetal Neonatal Med. 2015;28(16):1907–11.
Alpoim PN, et al. Polymorphisms in endothelial nitric oxide synthase gene in early and late severe preeclampsia. Nitric Oxide. 2014;42:19–23.
Chen Y, et al. Polymorphisms of the endothelial nitric oxide synthase gene in preeclampsia in a Han Chinese population. Gynecol Obstet Investig. 2014;77(3):150–5.
Rahimi Z, Aghaei A, Vaisi-Raygani A. Endothelial nitric oxide Synthase (eNOS) 4a/b and G894T polymorphisms and susceptibility to preeclampsia. J Reprod Infertil. 2013;14(4):184–9.
Dai B, et al. The polymorphism for endothelial nitric oxide synthase gene, the level of nitric oxide and the risk for pre-eclampsia: a meta-analysis. Gene. 2013;519(1):187–93.
Tulenko T, et al. The in vitro effect on arterial wall function of serum from patients with pregnancy-induced hypertension. Am J Obstet Gynecol. 1987;156(4):817–23.
Li F, et al. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol. 2012;23(4):652–60.
Zhu M, et al. Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2016;311(3):R505–21.
Haram K, Mortensen JH, Nagy B. Genetic aspects of preeclampsia and the HELLP syndrome. J Pregnancy. 2014;2014:910751.
• Berends AL, et al. STOX1 Gene in pre-eclampsia and intrauterine growth restriction. BJOG. 2007;114(9):1163–7. First study implicating the STOX gene as a hereditary basis for PE.
Rigourd V, et al. Re-evaluation of the role of STOX1 transcription factor in placental development and preeclampsia. J Reprod Immunol. 2009;82(2):174–81.
Kukor Z, Valent S, Toth M. Regulation of nitric oxide synthase activity by tetrahydrobiopterin in human placentae from normal and pre-eclamptic pregnancies. Placenta. 2000;21(8):763–72.
• Sankaralingam S, et al. Evidence for increased methylglyoxal in the vasculature of women with preeclampsia: role in upregulation of LOX-1 and arginase. Hypertension. 2009;54(4):897–904. Identifies a mechanism in which methylglyoxal increases arginase, and then LOX-1 expression in cultured endothelial cells, likely via uncoupling of eNOS. This is important since LOX-1 and arginase both contribute to oxidative stress, and are increased in PE.
Cominacini L, et al. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J Biol Chem. 2001;276(17):13750–5.
• Ahmed A, Ramma W. Unravelling the theories of pre-eclampsia: are the protective pathways the new paradigm? Br J Pharmacol. 2015;172(6):1574–86. A provocative review paper by the person who first proposed a role for sFlt-1 and reduced VEGF signaling in PE (in 1997).
Ozler A, et al. Serum levels of neopterin, tumor necrosis factor-alpha and Interleukin-6 in preeclampsia: relationship with disease severity. Eur Rev Med Pharmacol Sci. 2012;16(12):1707–12.
Kronborg CS, et al. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta Obstet Gynecol Scand. 2011;90(7):791–6.
Nayeri UA, et al. Antenatal corticosteroids impact the inflammatory rather than the antiangiogenic profile of women with preeclampsia. Hypertension. 2014;63(6):1285–92.
Harmon AC, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci. 2016;130(6):409–19.
Saleh L, et al. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther Adv Cardiovasc Dis. 2016;10(5):282–93.
Lankhorst S, Danser AH, van den Meiracker AH. Endothelin-1 and antiangiogenesis. Am J Physiol Regul Integr Comp Physiol. 2016;310(3):R230–4.
Jain A. Endothelin-1: a key pathological factor in pre-eclampsia? Reprod BioMed Online. 2012;25(5):443–9.
George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24(9):964–9.
• Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1288–95. A review that explains the big endothelin -NO story well, and provides a perspective on the potential importance of this physiological pathway under normal conditions vs. those of diminished NO bioavailability (such as PE).
Conrad KP. Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia. Semin Nephrol. 2011;31(1):15–32.
Bakrania B, et al. The Endothelin type a receptor as a potential therapeutic target in preeclampsia. Int J Mol Sci. 2017;18(3):E522.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Preeclampsia
Rights and permissions
About this article
Cite this article
Osol, G., Ko, N.L. & Mandalà, M. Altered Endothelial Nitric Oxide Signaling as a Paradigm for Maternal Vascular Maladaptation in Preeclampsia. Curr Hypertens Rep 19, 82 (2017). https://doi.org/10.1007/s11906-017-0774-6
Published:
DOI: https://doi.org/10.1007/s11906-017-0774-6