Abstract
Purpose of review
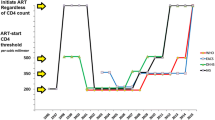
Optimal control of HIV can be achieved by early diagnosis followed by the initiation of antiretroviral therapy (ART). Two large randomised trials (TEMPRANO and START) have recently been published documenting the clinical benefits to HIV-positive adults of early ART initiation. Main findings are reviewed with a focus on serious non-AIDS (SNA) conditions.
Recent findings
Data from the two trials demonstrated that initiating ART early in the course of HIV infection resulted in marked reductions in the risk of opportunistic diseases and invasive bacterial infections. This indicates that HIV causes immune impairment in early infection that is remedied by controlling viral replication. Intriguingly, in START, a marked reduction in risk of cancers, both infection-related and unrelated types of cancers, was observed. Like the findings for opportunistic infections, this anti-cancer effect of early ART shows how the immune system influences important pro-oncogenic processes. In START, there was also some evidence suggesting that early ART initiation preserved kidney function, although the clinical consequence of this remains unclear. Conversely, while no adverse effects were evident, the trials did not demonstrate a clear effect on metabolic-related disease outcomes, pulmonary disease, or neurocognitive function.
Summary
HIV causes immune impairment soon after acquisition of infection. ART reverses this harm at least partially. The biological nature of the immune impairment needs further elucidation, as well as mechanisms and clinical impact of innate immune activation. Based on the findings from TEMPRANO and START, and because ART lowers the risk of onward transmission, ART initiation should be offered to all persons following their diagnosis of HIV.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–8. International cohort collaboration demonstrating marked reduction in last 15 years in fatal AIDS and SNA-related events incl those caused by cardiovascular and liver pathologies but not non-AIDS cancers. Whereas reduction in AIDS conditions were associated with gradually increasing CD4+ lymphocyte count, this was not the case for the SNA conditions, but perhaps better quality of care .
The Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96.
Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22(18):2409–18.
Lifson AR, Belloso WH, Davey RT, Duprez D, Gatell JM, Hoy JF, et al. Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV Clin Trials. 2010;11(4):205–19.
May MT, Vehreschild J, Trickey A, Obel N, Reiss P, Bonnet F, et al. Mortality according to CD4 count at start of combination antiretroviral therapy among HIV-infected patient followed for up to 15 years after start of treatment: collaborative cohort study. Clin Infect Dis. 2016;62:1571–7.
Baker JV, Peng G, Rapkin J, Krason D, Reilly C, Cavert WP, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J Acquir Immune Defic Syndr. 2008;48:541–6.
Achhra AC, Amin J, Law MG, Emery S, Gerstoft J, Gordin FM, et al. Immunodeficiency and the risk of serious clinical endpoints in a well studied cohort of treated HIV-infected patients. AIDS. 2010;24(12):1877–86.
•• Grund B, Baker JV, Deeks SG, et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One. 2016;11:e0155100. Combined cohort analysis demonstrating persisting prognostic impact from initial levels of interleukin-& and D-dimer. These observations were confirmed in the START cohort (Baker JV, Sharma S, Grund B, et al. Association of inflammation and coagulation with clinical risk in the START Trial. Abstract, Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washington, 2017) .
Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26.
Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1 infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63.
CASCADE Collaboration, Jonsson M, Fusco JS, Cole SR, Thomas JC, Porter K, et al. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–9.
Cain LE, Logan R, Robins JM, Sterne JA, Sabin C, Bansi L, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–15.
• The HIV-Causal Collaboration, Lodi S, Phillips A, Logan R, Olson A, Costagliola D, et al. Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV. 2015;2:e335-e-345. Most recent report to address whether statistical analysis of observational studies can predict the benefit from earlier initiation of ART. The predicted benefit (3–20%) was smaller than what was observed in the START study .
Lane HC, Neaton JD. When to start therapy for HIV infection: a swinging pendulum in search of data. Ann Intern Med. 2003;138(8):680–1.
Lundgren JD, Babiker AG, Gordin FM, et al. When to start antiretroviral therapy: the need for an evidence base during early HIV infection. BMC Med. 2013;11:148.
•• TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22. Single country (Ivory Coast) randomised controlled trial demonstrating clinical benefit from starting ART earlier rather than later in course of early HIV infection. Benefit was due to reduced risk of tuberculosis and invasive bacterial infections (44% reduction) .
•• The INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. Large, geographical diverse randomised controlled trial demonstrating clinical benefit from starting ART earlier rather than later in course of early HIV infection. Benefit was due to reduced risk of opportunistic infections and cancer (57%) .
INSIGHT Strategic Timing of AntiRetroviral Treatment (START) Study Group, Lundgren J, Babiker A, Gordin F, Emery S, Fätkenheuer G, et al. Why START? Reflections that led to the conduct of this large long-term strategic HIV trial. HIV Med. 2015;16(Suppl 1):1–9. https://doi.org/10.1111/hiv.12227.
Babiker AG, Emery S, Fätkenheuer G, et al. Considerations in the rationale, design and methods of the Strategic Timing of AntiRetroviral Treatment (START) study. Clin Trials. 2013;10(1 Suppl):S5–S36.
• Lodi S, Sharma S, Lundgren JD, Phillips AN, Cole SR, Logan R, et al. The per-protocol effect of immediate versus deferred antiretroviral therapy initiation. AIDS. 2016;30(17):2659–63. Analysis of the START cohort with aim to assess the benefit from earlier ART if all had all the trial participants adhered to the protocol. The ITT effect estimate (as reported in the N Engl J Med main article) may underestimate the benefit of immediate ART initiation by 23%.
Molina JM, Grund B, Gordin F, et al. Who benefited most from immediate treatment in START? A subgroup analysis. Abstract THAB0201, AIDS 2016, Durban, South Africa; 2016.
Lifson AR, Grund B, Gardner EM, Kaplan R, Denning E, Engen N, et al. Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection. AIDS. 2017;31(7):953–63.
• Babiker A, Grund B, Sharma S, et al. The role of HIV RNA and T cell counts, percent and ratio in explaining the benefit of immediate ART initiation in HIV+ individuals with high CD4+ counts. Abstract, AIDS 2016, Durban, South Africa; 2016. Analysis of the START study focusing on which immunological parameters could explain benefit from earlier ART initiation. Whereas changes in absolute CD4 count over follow-up only explained a small fraction of benefit (15%), changes in the CD4:CD8 ratio was a better predictor (45%).
Mocroft A, Furrer HJ, Miro JM, Reiss P, Mussini C, Kirk O, et al. The incidence of AIDS-defining illnesses at a current CD4 count ≥ 200 cells/μL in the post-combination antiretroviral therapy era. Clin Infect Dis. 2013;57(7):1038–47.
• O'Connor J, Vjecha MJ, Phillips AN, Angus B, Cooper D, Grinsztejn B, et al. Effect of immediate initiation of antiretroviral therapy on risk of severe bacterial infections in HIV-positive people with CD4 cell counts of more than 500 cells per μL: secondary outcome results from a randomised controlled trial. Lancet HIV. 2017;4(3):e105–12. Analysis in START cohort of how earlier ART reduced substantially (61%) the risk of contracting a composite endpoint of severe bacterial infection (including bacterial pneumonia, pulmonary or extrapulmonary tuberculosis, or any bacterial infectious disorder of grade 4 severity, that required unscheduled hospital admissions, or caused death). This benefit was partially explained by how earlier ART increased CD4+ lymphocyte count.
• Borges AH, Neuhaus J, Babiker AG, et al. Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clin Infect Dis. 2016;63:1668–76. Detailed analysis of cancer emerging in the START cohort, and how earlier initiation of ART reduced risk of both infectious-related and un-related types of cancers. Intriguingly, adjusted for time-updated HIV-RNA levels (but not CD4+ count) attenuated the observed benefit from earlier initiation of ART, but this effect was not observed for infectious-related, suggesting that the anti-cancer benefit from earlier ART initiation is not solely attributable to suppression of HIV replication.
Reekie J, Kosa C, Engsig F, Monforte AD, Wiercinska-Drapalo A, Domingo P, et al. Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer. 2010;116(22):5306–15.
Kersten MJ, Van Gorp J, Pals ST, Boon F, Van Oers MH. Expression of Epstein-Barr virus latent genes and adhesion molecules in AIDS-related non-Hodgkin’s lymphomas: correlation with histology and CD4-cell number. Leuk Lymphoma. 1998;30(5–6):515–24.
Arvey A, Ojesina AI, Pedamallu CS, Ballon G, Jung J, Duke F, et al. The tumor virus landscape of AIDS-related lymphomas. Blood. 2015;125(20):e14–22.
Mazzuca P, Marsico S, Schulze K, Mitola S, Pils MC, Giagulli C, Guzman CA, Caruso A, Caccuri F. Role of Autophagy in HIV-1 Matrix Protein p17-Driven Lymphangiogenesis. J Virol. 2017;91(16). https://doi.org/10.1128/JVI.00801-17.
Borges ÁH. Combination antiretroviral therapy and cancer risk. Curr Opin HIV AIDS. 2017;12(1):12–9.
• Friis-Møller N, Ryom L, Smith C, Weber R, Reiss P, Dabis F, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the dData-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–23. Updated version of the D:A:D study predictor of absolute underlying risk of cardiovascular disease in HIV+ populations. This and risk prediction tools for chronic kidney disease are available for online use at http://www.chip.dk/Tools .
Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. https://doi.org/10.1371/journal.pone.0044454.
Kuller LH, Tracy R, Belloso W, Wit SD, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203.
Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56:36–43.
Baker JV, Sharma S, Grund B, Rupert A, Metcalf JA, Schechter M, et al.Systemic Inflammation, Coagulation, and Clinical Risk in the START Trial. Open Forum Infect Dis. 2017;4(4):ofx262. http://www.ncbi.nlm.nih.gov/pubmed/29308409
• Baker JV, Sharma S, Achhra AC, Bernardino JI, Bogner JR, Duprez D, et al. Changes in cardiovascular disease risk factors with immediate versus deferred antiretroviral therapy initiation among HIV-positive participants in the START (Strategic Timing of Antiretroviral Treatment) Trial. J Am Heart Assoc. 2017;6(5). Analysis in START cohort of how earlier ART initiation impact traditional cardiovascular risk factors, iincluding worsening dyslipidaemia (in part depending on type of antiretroviral drug used), but decreased use of blood pressure medications, leading to an overall projected clinically insignificant impact on cardiovascular risk. Risk of new onset diabetes was not affected.
• Ghehi C, Gabillard D, Moh R, Badje A, Kouamé GM, Oouttara E, et al. High correlation between Framingham equations with BMI and with lipids to estimate cardiovascular risks score at baseline in HIV-infected adults in the Temprano trial, ANRS 12136 in Côte d’Ivoire. PLoS One. 2017;12(6):e0177440. Analysis of the TEMPRANO study using the Framingham equation, assessing temporal trends during follow-up and between the two randomised arms of the trial. No effect of earlier ART initiation affected this outcome.
• Baker JV, Hullsiek KH, Engen NW, Nelson R, Chetchotisakd P, Gerstoft J, et al. Early antiretroviral therapy at high CD4 counts does not improve arterial elasticity: a substudy of the Strategic Timing of AntiRetroviral Treatment (START) Trial. Open Forum Infect Dis. 2016;3(4):ofw213. Substudy in START assessing whether earlier ART affects changes in arterial elasticity (a marker of arterial wall disease which in general population studies are closely linked with risk of cardiovascular disease). No benefit from earlier ART was observed in this study.
Trullas JC, Mocroft A, Cofan F, Tourret J, Moreno A, Bagnis CI, et al. Dialysis and renal transplantation in HIV-infected patients: a European survey. J Acquir Immune Defic Syndr. 2010;55(5):582–9.
Ryom L, Lundgren JD, Ross M, Kirk O, Law M, Morlat P, et al. Renal impairment and cardiovascular disease in HIV-positive individuals: the D:A:D study. J Infect Dis. 2016;214(8):1212–20.
Parsa A, Kao L, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–96.
• Mocroft A, Lundgren JD, Ross M, Fux CA, Reiss P, Moranne O, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3(1):e23–32. International cohort study demonstrating dose-response association between duration of tenofovir disoproxil fumarate, ritonavir-boosted atazanavir, or ritonavir-boosted lopinavir usage and gradually increasing risk of developing chronic kidney disease. This finding did in relative terms not interact with patients underlying risk of contracting this outcome, implying that this complication is relatively common in those with elevated predicted underlying risk (i.e. a low number needed to harm).
Mocroft A, Lundgren J, Ross M, Law M, Reiss P, Kirk O, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med. 2015;12:e1001809.
Achhra AC, Mocroft A, Ross M, Ryom-Nielson L, Avihingsanon A, Bakowska E, et al. Impact of early versus deferred antiretroviral therapy on estimated glomerular filtration rate in HIV-positive individuals in the START trial. Int J Antimicrob Agents. 2017.
Kunisaki KM, Niewoehner DE, Collins G, Nixon DE, Tedaldi E, Akolo C, et al. Pulmonary function in an international sample of HIV-positive, treatment-naïve adults with CD4 counts > 500 cells/μL: a substudy of the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):119–28.
•• Kunisaki KM, Niewoehner DE, Collins G, Aagaard B, Atako NB, Bakowska E, et al. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir Med. 2016;4(12):980–9. Substudy from START study, assessing whether earlier ART affects longitudinal pulmonary function over a 2 year period. No such effect was found, irrespective of smoking status, which by itself markedly affected the course of pulmonary function.
Hoy J, Grund B, Roediger M, Ensrud KE, Brar I, Colebunders R, et al. Interruption or deferral of antiretroviral therapy reduces markers of bone turnover compared with continuous therapy: The SMART body composition substudy. J Bone Miner Res. 2013;28(6):1264–74.
• Hoy JF, Grund B, Roediger M, Schwartz AV, Shepherd J, Avihingsanon A, et al. Immediate initiation of antiretroviral therapy for HIV infection accelerates bone loss relative to deferring therapy: findings from the START bone mineral density substudy, a randomized trial. J Bone Miner Res. 2017;32:1945–55. https://doi.org/10.1002/jbmr.3183. Substudy from START study, assessing whether earlier ART affects changes in bone mineral density over a 2 year period. Earlier ART reduced density more so in the first but not the second year, and this initial decline was not linked to type of ART used.
Borges ÁH, Hoy J, Florence E, Sedlacek D, Stellbrink HJ, Uzdaviniene V, et al. Antiretrovirals, fractures, and osteonecrosis in a large international HIV cohort. Clin Infect Dis. 2017;64(10):1413–21.
Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, et al. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73(5):342–8.
Wright E, Grund B, Robertson K, Cysique L, Collins G, Brew B et al. No difference between the effects of immediate versus deferred ART on neuropsychological test performance in HIV-positive adults with CD4+ cell counts > 500 cells/μL. 15th European AIDS Conference (EACS), Barcelona, October 2015.
•• Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–9. Follow-up report to original HPTN 052 report documenting substantial reduction (93%) in risk of linked HIV transmission from placing HIV+ person on ART. Whereas no linked transmission was observed while index HIV+ person was on fully suppressive ART, a total of 8 linked transmissions were observed after initiation of ART stressing that full adherence is required.
•• Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using supportive antiretroviral therapy. JAMA. 2016;316:171–81. International study assessing the risk of HIV transmission in serodiscordant relationships having condom-less sex, and where the initial HIV+ person was on fully suppressive ART. No linked transmissions were observed after 40,000+ sexual intercourses. Supports claim that fully suppressive ART renders the person no longer able to transmit HIV.
Jean K, Boily MC, Danel C, Moh R, Badjé A, Desgrées-du-Loû A, et al. What level of risk compensation would offset the preventive effect of early antiretroviral therapy? Simulations from the TEMPRANO trial. Am J Epidemiol. 2016;184:755–60.
• Sereti I, Gulick RM, Krishnan S, et al. ART in HIV persons with pre-treatment viremia ≤ 3000 c/mL: the START study. Abstract 984, Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washington, 2017. Subgroup analysis of START cohort, focused on those entering the study with low HIV RNA viral load. Earlier ART raised CD4+ lymphocyte count and reduced risk of HIV viral rebound . No power was available to assess impact on clinical events from earlier initiation of ART.
The SPARTAC Trial Investigators, Fidler S, Porter K, Ewings F, Frater J, Ramjee G, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368:207–17.
Crowell TA, Hatano H. Clinical outcomes and antiretroviral therapy in ‘elite’ controllers: a review of the literature. J Virus Erad. 2015;1:72–7.
Borges AH, Neuhaus J, Sharma S, et al. Benefit of continuous/immediate ART on disease risk: SMART & START combined analysis. Abstract 793, Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washington; 2017.
Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, et al. Normalization of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational study. Lancet. 2007;370:407–13.
Acknowledgements
The authors would like to thank the INSIGHT (International Network for Strategic Initiatives in Global HIV Trials), its leadership including Drs. F Gordin, A Babiker, A Phillips, J Baker and B Grund, and main funder division of AIDS, National Institutes of Allergy and Infectious Diseases, USA.
Funding
This work was supported by the Danish National Research Foundation [grant 126].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alvaro H Borges is supported by Lundbeckfonden (grant R219-2016-762). James D. Neaton reports grants from NIH and NIAID. Jens D. Lundgren declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Co-infections and Comorbidity
Rights and permissions
About this article
Cite this article
Lundgren, J.D., Borges, A.H. & Neaton, J.D. Serious Non-AIDS Conditions in HIV: Benefit of Early ART. Curr HIV/AIDS Rep 15, 162–171 (2018). https://doi.org/10.1007/s11904-018-0387-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-018-0387-y