Abstract
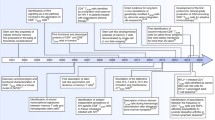
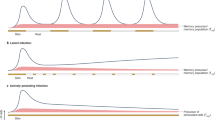
In analogy to many tissues in which mature, terminally differentiated cells are continuously replenished by the progeny of less differentiated, long-lasting stem cells, it has been suspected that memory T lymphocytes might contain small numbers of stem cell-like cells. However, only recently have such cells been physically identified and isolated from humans, mice, and nonhuman primates. These cells, termed “T memory stem cells” (TSCM), represent approximately 2–4 % of all circulating T lymphocytes, seem to be extremely durable, and can rapidly differentiate into more mature central memory, effector memory, and effector T cells, while maintaining their own pool size through homeostatic self-renewal. Although it is becoming increasingly evident that that these cells have critical roles for T cell homeostasis and maintaining life-long cellular immunity against microbial pathogens during physiological conditions, they also seem intrinsically involved in many key aspects of HIV/SIV disease pathogenesis. Current data suggest that CD4+ TSCM cells represent a core element of the HIV-1 reservoir in patients treated with suppressive antiretroviral therapy (ART) and that relative resistance of CD4+ TSCM cells to SIV represents a distinguishing feature of non-pathogenic SIV infection in natural hosts. This article summarizes recent studies investigating the role of TSCM in HIV/SIV infection.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203.
Restifo NP, Gattinoni L. Lineage relationship of effector and memory T cells. Curr Opin Immunol. 2013;25:556–63.
Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63.
Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7.
Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5.
Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35.
Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nat Rev Cancer. 2012;12:671–84.
Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–44.
Stemberger C et al. Stem cell-like plasticity of naive and distinct memory CD8+ T cell subsets. Semin Immunol. 2009;21:62–8.
Papatriantafyllou M. T cell memory: the stem of T cell memory. Nat Rev Immunol. 2011;11:716.
Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–8.
Gattinoni L et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7.
Gattinoni L et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–13.
Lugli E, et al. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–9. This is the first article to describe CD8+ and CD4+ T SCM cells in nonhuman primates.
Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–305.
Buzon MJ, et al. HIV-1 persistence in CD4 T cells with stem cell-like properties. Nat Med. 2014;20:139–42. This study first described the importance of CD4+ T SCM cells for long-term HIV-1 persistence in HIV-1-infected individuals treated with suppressive ART.
Cartwright EK, et al. Divergent CD4+ T memory stem cell dynamics in pathogenic and nonpathogenic simian immunodeficiency virus infections. J Immunol. 2014;192:4666–73. Here the authors provide the first evidence for a role of CD4+ T SCM in the pathogenesis of SIV infection. The main conclusion is that CD4+ T SCM cells are preferentially spared from SIV infection in the natural host sooty mangabeys.
Jaafoura S, et al. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4(+) memory T cells. Nat Commun. 2014;5:5407. This work independently confirmed the contribution of CD4+ T SCM cells to the long-term HIV-1 reservoir.
Lugli E et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2012;8:33–42.
Muranski P et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–85.
Graef P et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity. 2014;41:116–26.
Cieri N et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–84.
Ribeiro SP et al. The CD8+ memory stem T cell (TSCM) subset is associated with improved prognosis in chronic HIV-1 infection. J Virol. 2014;88:13836–44.
Ndhlovu ZM et al. Elite controllers with low to absent effector CD8+ T cell responses maintain highly functional, broadly directed central memory responses. J Virol. 2012;86:6959–69.
Cashin K et al. Differences in coreceptor specificity contribute to alternative tropism of HIV-1 subtype C for CD4+ T-cell subsets, including stem cell memory T-cells. Retrovirology. 2014;11:97.
Flynn JK et al. Quantifying susceptibility of CD4+ stem memory T-cells to infection by laboratory adapted and clinical HIV-1 strains. Viruses. 2014;6:709–26.
Tabler CO et al. CD4+ memory stem cells are infected by HIV-1 in a manner regulated in part by SAMHD1 expression. J Virol. 2014;88:4976–86.
Buzon MJ, et al. Long-term antiretroviral treatment initiated in primary HIV-1 infection affects the size, composition and decay kinetics of the reservoir of HIV-1 infected CD4 T cells. J Virol. 2014;88:10056–65. These authors show that early initiation of ART results in a smaller overall HIV-1 reservoir in CD4+ T SCM cells.
Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–39.
Valentine LE, Watkins DI. Relevance of studying T cell responses in SIV-infected rhesus macaques. Trends Microbiol. 2008;16:605–11.
Brenchley JM, Paiardini M. Immunodeficiency lentiviral infections in natural and non-natural hosts. Blood. 2011;118:847–54.
Pandrea IV et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179:3035–46.
Whitney JB et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014;512:74–7.
Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: showing AIDS the door. Science. 2012;335:1188–93.
Pandrea I et al. Paucity of CD4+ CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–76.
Paiardini M et al. Low levels of SIV infection in sooty mangabey central memory CD(4)(+) T cells are associated with limited CCR5 expression. Nat Med. 2011;17:830–6.
Chahroudi A et al. Target cell availability, rather than breast milk factors, dictates mother-to-infant transmission of SIV in sooty mangabeys and rhesus macaques. PLoS Pathog. 2014;10:e1003958.
Pandrea I et al. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J Virol. 2008;82:5501–9.
Pandrea I et al. Mucosal simian immunodeficiency virus transmission in African green monkeys: susceptibility to infection is proportional to target cell availability at mucosal sites. J Virol. 2012;86:4158–68.
Brenchley JM et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–8.
Okoye A et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–85.
Brenchley JM et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood. 2012;120:4172–81.
Descours B et al. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Infect Dis. 2012;54:1495–503.
Saez-Cirion A et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9:e1003211.
Klatt NR, et al. Limited HIV infection of central memory and stem cell memory CD4+ T cells is associated with lack of progression in viremic individuals. PLoS Pathog. 2014;10:e1004345. This study identified a fascinating subset of HIV-1-infected individuals, termed “viremic non-progressors”, that remain clinically healthy in the face of high viral loads. Interestingly, they also found low levels of HIV DNA in CD4+ T SCM cells from these patients, similar to what has been observed in sooty mangabeys.
Ring A, Kim YM, Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev. 2014;10:512–25.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205.
Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 2014;13:513–32.
Schilham MW et al. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–91.
Willinger T et al. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J Immunol. 2006;176:1439–46.
Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–45.
Persaud D et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369:1828–35.
Hutter G et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8.
Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–62.
Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8:97–106.
Wang J, Sullenger BA, Rich JN. Notch signaling in cancer stem cells. Adv Exp Med Biol. 2012;727:174–85.
Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105:1087–92.
Carter CC et al. HIV-1 utilizes the CXCR4 chemokine receptor to infect multipotent hematopoietic stem and progenitor cells. Cell Host Microbe. 2011;9:223–34.
McNamara LA, Collins KL. Hematopoietic stem/precursor cells as HIV reservoirs. Curr Opin HIV AIDS. 2011;6:43–8.
Josefsson L et al. Hematopoietic precursor cells isolated from patients on long-term suppressive HIV therapy did not contain HIV-1 DNA. J Infect Dis. 2012;206:28–34.
Durand CM et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J Infect Dis. 2012;205:1014–8.
Zhang J, Scadden DT, Crumpacker CS. Primitive hematopoietic cells resist HIV-1 infection via p21. J Clin Invest. 2007;117:473–81.
Acknowledgments
AC is supported by the American Foundation for AIDS Research (grant 108905-56-RGRL) and by an NICHD Child Health Research Career Development Award (K12 HD072245). ML is supported by the American Foundation for AIDS Research (grant 108302-51-RGRL), by the Doris Duke Charitable Foundation (grant 2009034), and by NIH grants AI098487 and AI106468. GS is supported by NIH grant R37AI066998.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Ann Chahroudi, Guido Silvestri, and Mathias Lichterfeld declare that they have patent pending on Wnt pathway inhibitors for treating viral infections.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on HIV Pathogenesis and Treatment
Rights and permissions
About this article
Cite this article
Chahroudi, A., Silvestri, G. & Lichterfeld, M. T Memory Stem Cells and HIV: a Long-Term Relationship. Curr HIV/AIDS Rep 12, 33–40 (2015). https://doi.org/10.1007/s11904-014-0246-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-014-0246-4