Abstract
Purpose of Review
Screening for hepatitis B viral infection in the USA is currently recommended for high-risk individuals and pregnant persons. Effective vaccines for hepatitis B have been available since the early 1980s, and have been recommended as part of the universal childhood immunization series since the 1990s.
Recent Findings
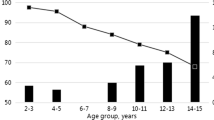
Vaccination rates remain low among the general population of persons who were not vaccinated in childhood or adolescence; despite efforts to increase adult vaccination rates, persons at highest risk of exposure—for example, injection drug users—may also be among those with low access to health care.
Summary
Although there is limited data to support universal screening for hepatitis B, newer vaccine regimens with fewer doses may address some challenges to improving rates of vaccination among US adults with and without risk factors for infection. The following review summarizes evolving recommendations for hepatitis B screening and vaccination in the USA.

Similar content being viewed by others
References
Viral hepatitis surveillance — United States. Centers for Disease Control and Prevention.
Public health reporting and national notification for acute hepatitis B infections. Council of State and Territorial Epidemiologists;Position statement 11-ID-03.
Roberts H, Ly KN, Yin S, Hughes E, Teshale E, Jiles R. Prevalence of hepatitis B virus (HBV) infection, vaccine-induced immunity, and susceptibility among at-risk populations: U.S. households, 2013-2018. Hepatology 2021.
Wong RJ, Brosgart CL, Welch S, Block T, Chen M, Cohen C, Kim WR, et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology. 2021.
Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, Donahue K, et al. Screening for hepatitis B virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. Jama. 2020;324:2415–22.
Tang LSYCE, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018;319:1802–13.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99.
David H. Spach M, Maria A. Corcorran, MD. HBV screening and diagnosis. Hepatitis B Online 2021.
Wong RJ, Brosgart CL, Welch S, Block T, Chen M, Cohen C, Kim WR, et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology. 2021;74:607–26.
Wolffram IPD, Bätz O, et al. German Check-Up 35+ Study Group. Prevalence of elevated ALT values, HBsAg, and anti-HCV in the primary care setting and evaluation of guideline defined hepatitis risk scenarios. J Hepatol. 2015;62:1256–64.
Bottero JBA, Lemoine M, et al. Current state of and needs for hepatitis B screening: results of a large screening study in a low prevalent, metropolitan region. PLoS One. 2014;9.
Spenatto NBS, Mularczyk M, et al. Hepatitis B screening: who to target? A French sexually transmitted infection clinic experience. J Hepatol. 2013;58:690–7.
Chou R, Blazina I, Bougatsos C, Holmes R, Selph S, Grusing S, Jou J. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;324:2423–36.
Patel EU, Thio CL, Boon D, Thomas DL, Tobian AAR. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011-2016. Clin Infect Dis. 2019;69:709–12.
Vijayadeva V, Spradling PR, Moorman AC, Rupp LB, Lu M, Gordon SC, Henkle E, et al. Hepatitis B virus infection testing and prevalence among Asian and Pacific Islanders. Am J Manag Care. 2014;20:e98–e104.
Hu KQ, Pan CQ, Goodwin D. Barriers to screening for hepatitis B virus infection in Asian Americans. Dig Dis Sci. 2011;56:3163–71.
Toy M, Hutton D, Harris AM, Nelson N, Salomon JA, So S. Cost-effectiveness of 1-time universal screening for chronic hepatitis B infection in adults in the United States. Clin Infect Dis. 2022;74:210–7.
Sablan BPKD, Barzaga NG, et al. Demonstration of safety and enhanced seroprotection against hepatitis B with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine. 2012;30:2689–96.
Hyer RMD, Xing B, Jackson S, Janssen R. Safety of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant in adults. Vaccine. 2018;36:2604–11.
Martha C, Sanchez M. Hepatitis A and B vaccination in the United States, vaccine updates. Rhode Island Medical Journal. 2020.
Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1–31.
Schillie S VC, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep:1-31.
Jilg WSM, Deinhardt F. Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis. 160(5):766–9.
Alonso S, Guerra AR, Carreira L, Ferrer JA, Gutierrez ML, Fernandez-Rodriguez CM. Upcoming pharmacological developments in chronic hepatitis B: can we glimpse a cure on the horizon? BMC Gastroenterol. 2017;17:168.
Organization WH. Global health sector strategy on viral hepatitis 2016–2021. June 2016.
Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of hepatitis B virus infection and impact of vaccination on disease. Clin Liver Dis. 2016;20:607–28.
Rosen HR, Ghany MG, Chung RT, Lok ASF. NAM 2017 report: a national plan to eliminate hepatitis B and C in the United States by 2030 and the AASLD’s response. Hepatology. 2017;66:1020–2.
Group TNHBCSPW. Strategic plan for trans-NIH research to cure hepatitis B. Novmeber. 2019.
Atlanta: US Department of Health and Human Services CfDCaP. Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2017.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
Stuart C. Gordon receives grant/research support from AbbVie Pharmaceuticals, Bristol-Myers Squibb, Conatus, CymaBay, Exalenz BioScience, Gilead Pharmaceuticals, Intercept Pharmaceuticals, and Merck.
Other authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hepatitis B
Rights and permissions
About this article
Cite this article
Shamaa, O., Mendiratta, V., Trudeau, S. et al. Evolving Screening and Vaccination Recommendations for Hepatitis B in the USA. Curr Hepatology Rep 21, 37–43 (2022). https://doi.org/10.1007/s11901-022-00583-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-022-00583-3