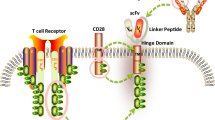
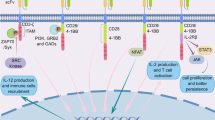
Abstract
Purpose of Review
We describe the significant technological leap from bench to bedside that was achieved through a strong academic-industry collaboration between dedicated clinicians and researchers at the University of Pennsylvania, the Children’s Hospital of Philadelphia, and Novartis to commercialize the chimeric antigen receptor T cell (CAR-T) therapy tisagenlecleucel (CTL019; Kymriah®; Novartis Pharma AG, Basel, Switzerland).
Recent Findings
Tisagenlecleucel was the first CAR-T therapy and the first gene therapy to receive US Food and Drug Administration approval in 2017, with an initial indication for pediatric and young adult patients with relapsed or refractory (r/r) acute lymphoblastic leukemia, followed by approval in May 2018 for a second indication in adult patients with r/r diffuse large B cell lymphoma. Subsequent approvals in the European Union, Switzerland, and Canada soon followed.
Summary
The tisagenlecleucel success story represents the development and commercialization of a first-of-its-kind personalized cellular therapy with a manufacturing process that supports commercial production and ongoing global clinical trials in a growing number of countries.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Kymriah (tisagenlecleucel) suspension for intravenous infusion [package insert]. East Hanover, NJ: Novartis Pharmceuticals Corporation; 2018.
Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–33. https://doi.org/10.1056/NEJMoa1103849.
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. https://doi.org/10.1126/scitranslmed.3002842.
June CH. Chimeric antigen receptors: engineering designer T cells for cancer therapy. Presented at: 2012 American Society of Clinical Oncology annual meeting, June 5, 2012; Chicago, IL. Available at: https://meetinglibrary.asco.org/record/68679/video. Accessed November 7, 2018.
Penn Medicine. University of Pennsylvania and Novartis form Alliance to expand use of personalized T cell therapy for Cancer patients [press release]. Available at: https://www.pennmedicine.org/news/news-releases/2012/august/university-of-pennsylvania-and. Accessed November 7, 2018. Philadelphia, PA: Penn Medicine; 2012.
Penn Medicine. Novartis-Penn Center for advanced cellular therapeutics unveiled at Penn medicine [press release]. Available at: https://www.pennmedicine.org/news/news-releases/2016/february/novartispenn-center-for-advanc. Accessed November 7, 2018. Philadelphia, PA: Penn Medicine; 2016.
United States Adopted Names Council. Tisagenleceleucel (BC-84). Statement on a nonproprietary name adopted by the USAN council. March 29, 2017. Available at: https://searchusan.ama-assn.org/undefined/documentDownload?uri=%2Funstructured%2Fbinary%2Fusan%2Ftisagenlecleucel.pdf. Accessed November 7, 2018.
Lebwohl D. KYMRIAH (tisagenlecleucel) case study: CD-19-directed genetically modified autologous T-cell immunotherapy for pediatric acute lymphoblastic leukemia (ALL). Presented at: Accelerating Anticancer Agent Development Validation Workshop; May 2-4, 2018.
Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92–101. https://doi.org/10.1016/j.omtm.2016.12.006.
Blanc V, Bousseau A, Caron A, Carrez C, Lutz RJ, Lambert JM. SAR3419: an anti-CD19-maytansinoid immunoconjugate for the treatment of B-cell malignancies. Clin Cancer Res. 2011;17(20):6448–58. https://doi.org/10.1158/1078-0432.CCR-11-0485.
Clinicaltrials.gov. Pilot study of redirected autologous T-cells engineered to contain anti-CD19 attached to TCR and 4-1BB signaling domains in patient with chemotherapy resistant or refractory CD19+ leukemia and lymphoma. NCT01029366. Available at: https://clinicaltrials.gov/ct2/show/NCT01029366. Accessed November 7, 2018.
Clinicaltrials.gov. Pilot study of donor lymphocyte infusions using donor T cells engineered to contain anti-CD19 attached to TCR And 4-1BB signaling domains in patients with relapsed CD19+ ALL after allogeneic stem cell transplantation. NCT01551043. Available at: https://clinicaltrials.gov/ct2/show/NCT01551043. Accessed November 7, 2018.
Clinicaltrials.gov. CHP 959 - A phase I/IIA study of redirected autologous T cells engineered to contain anti-CD19 attached to TCRzeta and 4-1BB signaling domains in patients with chemotherapy resistant or refractory CD19+ leukemia and lymphoma. NCT01626495. Available at: https://clinicaltrials.gov/ct2/show/NCT01626495. Accessed November 7, 2018.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. https://doi.org/10.1056/NEJMoa1407222.
Clinicaltrials.gov. A phase II, single arm, multicenter trial to determine the efficacy and safety of CTL019 in pediatric patients with relapsed and refractory B-cell acute lymphoblastic leukemia. NCT02228096. Available at: https://clinicaltrials.gov/ct2/show/NCT02228096. Accessed November 7, 2018.
Clinicaltrials.gov. A phase II, single arm, multicenter trial to determine the efficacy and safety of CTL019 in pediatric patients with relapsed and refractory B-cell acute lymphoblastic leukemia. NCT02435849. Available at: https://clinicaltrials.gov/ct2/show/NCT02435849. Accessed November 7, 2018.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–54. https://doi.org/10.1056/NEJMoa1708566.
Clinicaltrials.gov. A phase II, single arm, multicenter trial to determine the efficacy and safety of CTL019 in adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). NCT02445248. Available at: https://clinicaltrials.gov/ct2/show/NCT02445248. Accessed November 7, 2018.
•• Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. https://doi.org/10.1056/NEJMoa1709866 Publication of the ELIANA clinical trial, which led to regulatory approval of tisagenlecleucel for pediatric and young adult patients with acute lymphoblastic leukemia.
•• Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2018 [in press]. Publication of the JULIET clinical trial, which led to regulatory approval of tisagenlecleucel for adult patients with diffuse large B-cell lymphoma.
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. https://doi.org/10.1056/NEJMoa1215134.
Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168(4):724–40. https://doi.org/10.1016/j.cell.2017.01.016.
Novartis Pharmaceuticals Corporation. Novartis CAR-T cell therapy CTL019 unanimously (10-0) recommended for approval by FDA advisory committee to treat pediatric, young adult r/r B-cell ALL [press release]. Available at: https://www.novartis.com/news/media-releases/novartis-car-t-cell-therapy-ctl019-unanimously-10-0-recommended-approval-fda. Accessed November 7, 2018. East Hanover, NJ: Novartis Pharmaceuticals Corporation.
Bach PB. National coverage analysis of CAR-T therapies - policy, evidence, and payment. N Engl J Med. 2018;379(15):1396–8. https://doi.org/10.1056/NEJMp1807382.
Hao Y, Eldjerou L, Yang H, Qi CZ, Globe D. Cost-effectiveness analysis of Kymriah™ for the treatment of pediatric and young adult patients with relapsed or refractory B-Cell acute lymphoblastic leukemia in the United States. Presented at: American Society of Hematology Annual Meeting; December 9–12, 2017; Atlanta, GA. Abstract 609.
Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J Hematol Oncol. 2018;11(1):141. https://doi.org/10.1186/s13045-018-0681-6.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8. https://doi.org/10.1038/nm.4441.
Qin H, Ramakrishna S, Nguyen S, Fountaine TJ, Ponduri A, Stetler-Stevenson M, et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol Ther Oncolytics. 2018;11:127–37. https://doi.org/10.1016/j.omto.2018.10.006.
Cho JH, Okuma A, Al-Rubaye D, Intisar E, Junghans RP, Wong WW. Engineering Axl specific CAR and SynNotch receptor for cancer therapy. Sci Rep. 2018;8(1):3846. https://doi.org/10.1038/s41598-018-22252-6.
Hartmann J, Schussler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9(9):1183–97. https://doi.org/10.15252/emmm.201607485.
Yeku OO, Purdon T, Spriggs DR, Brentjens RJ. Interleukin-12 armored chimeric antigen receptor (CAR) T cells for heterogeneous antigen-expressing ovarian cancer. J Clin Oncol. 2018;36(5_suppl):12. https://doi.org/10.1200/JCO.2018.36.5_suppl.12.
Majors BS, Spencer T, Ericson SG, Romanov V. Initial experience in US commercial manufacturing of tisagenlecleucel, a chimeric antigen receptor (CAR)-T cell therapy for pediatric relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Presented at: 23rd European Hematology Association Meeting; June 14–17, 2018; Stockholm, Sweden. Abstract PS1156.
Acknowledgments
The authors would like to thank the following institutions and people for their valuable contributions to the development of the Novartis CAR-T cell program: The University of Pennsylvania, The Children’s Hospital of Philadelphia, clinical trial investigators, patients and families, Herve Hoppenot, Manuel Lichtman, David Epstein, Samuele Butera, Pascal Touchon, Serge Runser, Margit Jeschke, Karen Thudium, Jens Hasskarl, Patricia Wood, Ewelina Morawa, Lida Pacaud, Chris Keir, Spencer Fisk, Carl June, Stephan Grupp, Shannon Maude, Stephen Schuster, David Porter, David Teachey, Bruce Levine, William Chou, Stefanie Possekel, Mark Fishman, Bill Sellers, Allessandro Riva, Joe Jimenez, Juan Andres, Richard Tarapata, Michael Christiano, Nancy Griffin, Chris Klee, Chuck Wilson, Jennifer Brogdon, Abhijit Agarwal, Matthew Robson, Yanni Hao, Yan Lui, Jie Zhang, Kristen Harrington-Smith, Julie Millon, Karen Russo, Elaine Harney, Beth Ostergaard-Stillwell, Mark Morton, Clifford Baum, Sweta Shah, Kathryn Lund, Angela Shen, Marty Giedlin, Andrew Trovato, Kathrin Jinivizian, Charlene Hall, Lisa Haystrand, Rachelle Senzon, Maanasa Gowda, Lan Cao, Jason Hamilton, Narin Ahmed, Viera Muzithras, Nandita Shangari, Lori Tomassian.
Editorial assistance was provided Michael Hobert, PhD, of ProEd Communications, Inc., and was supported by Novartis Pharmaceuticals Corporation.
Funding
Editorial assistance was supported by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Stacie Ittershagen, Lamis Eldjerou, Eric Bleickardt, Manisha Patel, Oezlem Anak, Charlene Hall, Mimi Leung, Ali Shojaee, Deborah Roccoberton, Miriam Fuchs, and Florence Salmon declare that they are employees of Novartis Pharmaceuticals Corporation.
Solveig Ericson, Tetiana Taran, Vadim Romanov, and David Lebwohl declare that they are employees and shareholders of Novartis Pharmaceuticals Corporation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stem Cell Transplantation
Electronic Supplementary Material
ESM 1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Ittershagen, S., Ericson, S., Eldjerou, L. et al. Industry’s Giant Leap Into Cellular Therapy: Catalyzing Chimeric Antigen Receptor T Cell (CAR-T) Immunotherapy. Curr Hematol Malig Rep 14, 47–55 (2019). https://doi.org/10.1007/s11899-019-0498-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-019-0498-6