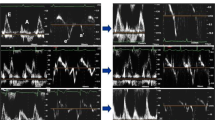
Abstract
Purpose of Review
This review summarizes recent developments highlighting the clinical utility of diastolic stress testing along the heart failure continuum.
Recent Findings
Invasive hemodynamic assessment of cardiac filling pressures during physiological stress is the gold-standard technique for unmasking diastolic dysfunction. Non-invasive surrogate techniques, such as Doppler ultrasound, have shown excellent agreement with invasive approaches and are now recommended by the American Society of Echocardiography and the European Association of Cardiovascular Imaging. While cycle exercise is often advocated, recent evidence supports the use of isometric handgrip as a viable alternative stressor.
Summary
Diastolic stress testing is a powerful tool to enhance detection of diastolic dysfunction, is able to differentiate between cardiac and non-cardiac pathology, and should be incorporated into routine clinical assessment.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–9. https://doi.org/10.1093/eurheartj/ehq426.
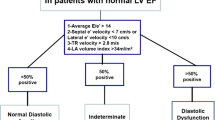
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–60. https://doi.org/10.1093/ehjci/jew082.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011.
Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47(9):1891–900. https://doi.org/10.1016/j.jacc.2006.02.042.
Borlaug BA. Exercise haemodynamics and outcome in patients with dyspnoea. Eur Heart J. 2014;35(44):3085–7. https://doi.org/10.1093/eurheartj/ehu350.
Mitter SS, Shah SJ, Thomas JD. A test in context: E/A and E/e’ to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol. 2017;69(11):1451–64. https://doi.org/10.1016/j.jacc.2016.12.037.
Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015;21(2):183–94. https://doi.org/10.1016/j.cmet.2015.01.005.
Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95(2):313–5.
Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953–9. https://doi.org/10.1056/NEJMoa032566.
van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3(6):e001293. https://doi.org/10.1161/JAHA.114.001293.
Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, Priebe L, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98(10):969–76.
Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, et al. STIM1-dependent store-operated Ca(2)(+) entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol. 2012;52(1):136–47. https://doi.org/10.1016/j.yjmcc.2011.11.003.
Goonasekera SA, Hammer K, Auger-Messier M, Bodi I, Chen X, Zhang H, et al. Decreased cardiac L-type Ca(2)(+) channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122(1):280–90. https://doi.org/10.1172/JCI58227.
Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75(3):434–42.
Sipido KR, Volders PG, Vos MA, Verdonck F. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc Res. 2002;53(4):782–805.
Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113(17):2089–96. https://doi.org/10.1161/CIRCULATIONAHA.105.573865.
Gonzalez A, Lopez B, Querejeta R, Zubillaga E, Echeverria T, Diez J. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension. 2010;55(6):1418–24. https://doi.org/10.1161/HYPERTENSIONAHA.109.149112.
Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131(6):550–9. https://doi.org/10.1161/CIRCULATIONAHA.114.009625.
Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, et al. PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105(7):631–8, 17 p following 8. https://doi.org/10.1161/CIRCRESAHA.109.198465.
Hidalgo C, Granzier H. Tuning the molecular giant titin through phosphorylation: role in health and disease. Trends Cardiovasc Med. 2013;23(5):165–71. https://doi.org/10.1016/j.tcm.2012.10.005.
Motoki H, Alraies MC, Dahiya A, Saraiva RM, Hanna M, Marwick TH, et al. Changes in left atrial mechanics following pericardiectomy for pericardial constriction. J Am Soc Echocardiogr. 2013;26(6):640–8. https://doi.org/10.1016/j.echo.2013.02.014.
Yamamoto K, Masuyama T, Tanouchi J, Uematsu M, Doi Y, Naito J, et al. Decreased and abnormal left ventricular filling in acute heart failure: role of pericardial constraint and its mechanism. J Am Soc Echocardiogr. 1992;5(5):504–14.
Borlaug BA, Carter RE, Melenovsky V, DeSimone CV, Gaba P, Killu A, et al. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10(4):e003612. https://doi.org/10.1161/CIRCHEARTFAILURE.116.003612.
Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, et al. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110(13):1799–805. https://doi.org/10.1161/01.CIR.0000142863.71285.74.
van Empel VP, Kaye DM, Borlaug BA. Effects of healthy aging on the cardiopulmonary hemodynamic response to exercise. Am J Cardiol. 2014;114(1):131–5. https://doi.org/10.1016/j.amjcard.2014.04.011.
Hollingsworth KG, Blamire AM, Keavney BD, Macgowan GA. Left ventricular torsion, energetics, and diastolic function in normal human aging. Am J Physiol Heart Circ Physiol. 2012;302(4):H885–92. https://doi.org/10.1152/ajpheart.00985.2011.
•• Samuel TJ, Beaudry R, Haykowsky MJ, Sarma S, Nelson MD. Diastolic stress testing: similarities and differences between isometric handgrip and cycle echocardiography. J Appl Physiol (1985). 2018; https://doi.org/10.1152/japplphysiol.00304.2018. This was the first study to demonstrate that isometric handgrip exercise has comparable utility at unmasking diastolic dysfunction measured non-invasively using echocardiography, compared to more commonly used cycle exercise.
• Samuel TJ, Beaudry R, Haykowsky MJ, Sarma S, Park S, Dombrowsky T, et al. Isometric handgrip echocardiography: a noninvasive stress test to assess left ventricular diastolic function. Clin Cardiol. 2017;40(12):1247–55. https://doi.org/10.1002/clc.22818. This was the first study to demonstrate that non-invasive isometric handgrip exercise can successfully differentiate between normal and abnormal diastolic function in a group of individuals at risk for heart failure (ACC/AHA stage A).
Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr. 2005;18(1):63–8. https://doi.org/10.1016/j.echo.2004.08.033.
Lewis BM, Houssay HE, Haynes FW, Dexter L. The dynamics of both right and left ventricles at rest and during exercise in patients with heart failure. Circ Res. 1953;1(4):312–20.
Levine BD, Lane LD, Buckey JC, Friedman DB, Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84(3):1016–23.
Drazner MH, Prasad A, Ayers C, Markham DW, Hastings J, Bhella PS, et al. The relationship of right- and left-sided filling pressures in patients with heart failure and a preserved ejection fraction. Circ Heart Fail. 2010;3(2):202–6. https://doi.org/10.1161/CIRCHEARTFAILURE.108.876649.
Levine BD. Regulation of central blood volume and cardiac filling in endurance athletes: the Frank-Starling mechanism as a determinant of orthostatic tolerance. Med Sci Sports Exerc. 1993;25(6):727–32.
Popovic ZB, Prasad A, Garcia MJ, Arbab-Zadeh A, Borowski A, Dijk E, et al. Relationship among diastolic intraventricular pressure gradients, relaxation, and preload: impact of age and fitness. Am J Physiol Heart Circ Physiol. 2006;290(4):H1454–9. https://doi.org/10.1152/ajpheart.00902.2005.
Shibata S, Hastings JL, Prasad A, Fu Q, Bhella PS, Pacini E, et al. Congestive heart failure with preserved ejection fraction is associated with severely impaired dynamic Starling mechanism. J Appl Physiol (1985). 2011;110(4):964–71. https://doi.org/10.1152/japplphysiol.00826.2010.
Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, et al. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012;47(8):565–72. https://doi.org/10.1016/j.exger.2012.04.006.
Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127(1):55–62. https://doi.org/10.1161/CIRCULATIONAHA.112.111302.
Fujimoto N, Shibata S, Hastings JL, Carrick-Ranson G, Bhella PS, Palmer D, et al. Effects of pericardial constraint and ventricular interaction on left ventricular hemodynamics in the unloaded heart. Am J Physiol Heart Circ Physiol. 2011;300(5):H1688–95. https://doi.org/10.1152/ajpheart.01198.2010.
Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588–95. https://doi.org/10.1161/CIRCHEARTFAILURE.109.930701.
Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97(12):964–9. https://doi.org/10.1136/hrt.2010.212787.
Andersen MJ, Ersboll M, Bro-Jeppesen J, Gustafsson F, Hassager C, Kober L, et al. Exercise hemodynamics in patients with and without diastolic dysfunction and preserved ejection fraction after myocardial infarction. Circ Heart Fail. 2012;5(4):444–51. https://doi.org/10.1161/CIRCHEARTFAILURE.112.967919.
Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J Am Coll Cardiol. 2010;55(16):1701–10. https://doi.org/10.1016/j.jacc.2009.11.076.
Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15(7):776–85. https://doi.org/10.1093/eurjhf/hft026.
Andersen MJ, Borlaug BA. Invasive hemodynamic characterization of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10(3):435–44. https://doi.org/10.1016/j.hfc.2014.03.001.
Andersen MJ, Ersboll M, Bro-Jeppesen J, Moller JE, Hassager C, Kober L, et al. Relationships between biomarkers and left ventricular filling pressures at rest and during exercise in patients after myocardial infarction. J Card Fail. 2014;20(12):959–67. https://doi.org/10.1016/j.cardfail.2014.09.012.
Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8(1):41–8. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001731.
Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ J. 2014;78(1):20–32.
Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37(43):3293–302. https://doi.org/10.1093/eurheartj/ehw241.
Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2015;66(15):1672–82. https://doi.org/10.1016/j.jacc.2015.07.067.
Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res. 2016;119(7):880–6. https://doi.org/10.1161/CIRCRESAHA.116.309184.
Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–47. https://doi.org/10.1161/CIRCULATIONAHA.106.632745.
Borlaug BA, Reddy YN. Determinants and correlates of exercise capacity in heart failure. JACC Heart Fail. 2015;3(10):815–7. https://doi.org/10.1016/j.jchf.2015.07.005.
Gharacholou SM, Scott CG, Borlaug BA, Kane GC, McCully RB, Oh JK, et al. Relationship between diastolic function and heart rate recovery after symptom-limited exercise. J Card Fail. 2012;18(1):34–40. https://doi.org/10.1016/j.cardfail.2011.09.010.
Hussain I, Mohammed SF, Forfia PR, Lewis GD, Borlaug BA, Gallup DS, et al. Impaired right ventricular-pulmonary arterial coupling and effect of sildenafil in heart failure with preserved ejection fraction: an ancillary analysis from the phosphodiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (RELAX) trial. Circ Heart Fail. 2016;9(4):e002729. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002729.
Kaye D, Shah SJ, Borlaug BA, Gustafsson F, Komtebedde J, Kubo S, et al. Effects of an interatrial shunt on rest and exercise hemodynamics: results of a computer simulation in heart failure. J Card Fail. 2014;20(3):212–21. https://doi.org/10.1016/j.cardfail.2014.01.005.
Lam CS, Grewal J, Borlaug BA, Ommen SR, Kane GC, McCully RB, et al. Size, shape, and stamina: the impact of left ventricular geometry on exercise capacity. Hypertension. 2010;55(5):1143–9. https://doi.org/10.1161/HYPERTENSIONAHA.109.146845.
Little WC, Borlaug BA. Exercise intolerance in heart failure with preserved ejection fraction: what does the heart have to do with it? Circ Heart Fail. 2015;8(2):233–5. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001966.
Mohammed SF, Borlaug BA, McNulty S, Lewis GD, Lin G, Zakeri R, et al. Resting ventricular-vascular function and exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7(4):580–9. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001192.
Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, et al. PhosphdiesteRasE-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (RELAX) trial: rationale and design. Circ Heart Fail. 2012;5(5):653–9. https://doi.org/10.1161/CIRCHEARTFAILURE.112.969071.
Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–77. https://doi.org/10.1001/jama.2013.2024.
Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J, et al. Resting and exercise haemodynamics in relation to six-minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2017;20:715–22. https://doi.org/10.1002/ejhf.976.
Zakeri R, Borlaug BA, McNulty SE, Mohammed SF, Lewis GD, Semigran MJ, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail. 2014;7(1):123–30. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000568.
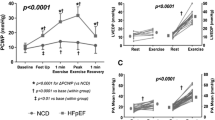
•• Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. The role of diastolic stress testing in the evaluation for HFpEF: a simultaneous invasive-echocardiographic study. Circulation. 2016; https://doi.org/10.1161/CIRCULATIONAHA.116.024822. This study confirmed the close relationship between invasively measured PCWP and non-invasive Doppler-derived E/e’ ratio in a large group of HFpEF patients ( n = 50). This study also demonstrated that low intensity cycle exercise (20W) was sufficient to elicit clinically significant increases in PCWP and E/e’ and was therefore formed a rationale for the use of low intensity exercise of many subsequent investigations.
Ha JW, Choi EY, Choi D, Park S, Shim CY, Lee JH, et al. Time course of recovery of left ventricular filling pressure after exercise in healthy subjects. Circ J. 2008;72(2):186–8.
Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89(4):372–83.
Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117(16):2051–60. https://doi.org/10.1161/CIRCULATIONAHA.107.716886.
Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–20.
• Rommel KP, von Roeder M, Oberueck C, Latuscynski K, Besler C, Blazek S, et al. Load-independent systolic and diastolic right ventricular function in heart failure with preserved ejection fraction as assessed by resting and handgrip exercise pressure-volume loops. Circ Heart Fail. 2018;11(2):e004121. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004121. This study was one of the first invasive investigations to utilize isometric handgrip exercise to elicit significant upward and leftward shift in the left and right ventricular end-diastolic pressure volume relationship, a hallmark of diastolic dysfunction, in a population of HFpEF patients.
Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57(3):461–9.
Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res. 1995;76(1):127–31.
Victor RG, Vissing SF, Urias L, Scherrer U. Central motor command activates sympathetic outflow to skin during static exercise in humans. Clin Res. 1989;37(2):A524-A.
Delaney EP, Greaney JL, Edwards DG, Rose WC, Fadel PJ, Farquhar WB. Exaggerated sympathetic and pressor responses to handgrip exercise in older hypertensive humans: role of the muscle metaboreflex. Am J Physiol Heart Circ Physiol. 2010;299(5):H1318–27. https://doi.org/10.1152/ajpheart.00556.2010.
Ogoh S, Wasmund WL, Keller DM, O-Yurvati A, Gallagher KM, Mitchell JH, et al. Role of central command in carotid baroreflex resetting in humans during static exercise. J Physiol Lond. 2002;543(1):349–64. https://doi.org/10.1113/jphysiol.2002.019943.
Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51(11):1112–9. https://doi.org/10.1016/j.jacc.2007.12.014.
Balderas-Villalobos J, Molina-Munoz T, Mailloux-Salinas P, Bravo G, Carvajal K, Gomez-Viquez NL. Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol. 2013;305(9):H1344–53. https://doi.org/10.1152/ajpheart.00211.2013.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–29. https://doi.org/10.1038/nrm1155.
Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61(1):70–6.
Hunter WC. Role of myofilaments and calcium handling in left ventricular relaxation. Cardiol Clin. 2000;18(3):443–57.
Shim CY, Park S, Choi EY, Hong GR, Choi D, Jang Y, et al. The relationship between ventricular-vascular uncoupling during exercise and impaired left ventricular longitudinal functional reserve in hypertensive patients. J Am Soc Hypertens. 2013;7(3):198–205. https://doi.org/10.1016/j.jash.2013.01.005.
Gibby C, Wiktor DM, Burgess M, Kusunose K, Marwick TH. Quantitation of the diastolic stress test: filling pressure vs. diastolic reserve. Eur Heart J Cardiovasc Imaging. 2013;14(3):223–7. https://doi.org/10.1093/ehjci/jes078.
Sonaglioni A, Lombardo M, Baravelli M, Trotta G, Sommese C, Anza C. Exercise stress echocardiography with tissue Doppler imaging in risk stratification of mild to moderate aortic stenosis. Int J Cardiovasc Imaging. 2015;31(8):1519–27. https://doi.org/10.1007/s10554-015-0724-9.
Christensen NL, Dahl JS, Carter-Storch R, Bakkestrom R, Jensen K, Steffensen FH, et al. Association between left atrial dilatation and invasive hemodynamics at rest and during exercise in asymptomatic aortic stenosis. Circ Cardiovasc Imaging. 2016;9(10) https://doi.org/10.1161/CIRCIMAGING.116.005156.
Obokata M, Nagata Y, Kado Y, Kurabayashi M, Otsuji Y, Takeuchi M. Ventricular-arterial coupling and exercise-induced pulmonary hypertension during low-level exercise in heart failure with preserved or reduced ejection fraction. J Card Fail. 2017;23(3):216–20. https://doi.org/10.1016/j.cardfail.2016.10.001.
Kosmala W, Przewlocka-Kosmala M, Rojek A, Marwick TH. Comparison of the diastolic stress test with a combined resting echocardiography and biomarker approach to patients with exertional dyspnea: diagnostic and prognostic implications. JACC Cardiovasc Imaging. 2018; https://doi.org/10.1016/j.jcmg.2017.10.008.
Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018; https://doi.org/10.1093/eurheartj/ehy331.
Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, et al. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2018;72(1):29–40. https://doi.org/10.1016/j.jacc.2018.04.039.
Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018; https://doi.org/10.1093/eurheartj/ehy268.
Hieda M, Howden E, Shibata S, Tarumi T, Lawley J, Hearon C Jr, et al. Preload-corrected dynamic Starling mechanism in patients with heart failure with preserved ejection fraction. J Appl Physiol (1985). 2018;124(1):76–82. https://doi.org/10.1152/japplphysiol.00718.2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pathophysiology: Neuroendocrine, Vascular, and Metabolic Factors
Rights and permissions
About this article
Cite this article
Samuel, T.J., Beaudry, R., Sarma, S. et al. Diastolic Stress Testing Along the Heart Failure Continuum. Curr Heart Fail Rep 15, 332–339 (2018). https://doi.org/10.1007/s11897-018-0409-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-018-0409-5