Abstract
Purpose of Review
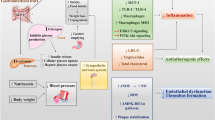
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) have positive effects on weight loss, blood pressure, hyperlipidemia, and glycemic control. They exhibit a broad range of effects on the cardiovascular system that are independent of changes in blood glucose. Cardiovascular outcome trials have demonstrated safety of GLP-1 RAs but results for cardiovascular efficacy were varied. The aim of the present review is the assessment of the effects of GLP-1 RAs on cardiovascular risk factors, and major cardiovascular events.
Recent Findings
Use of GLP-1 RAs was associated with relative risk reduction in cardiovascular mortality and all-cause mortality with no significant differences for the incidence of severe hypoglycemia, pancreatitis, pancreatic cancer, or medullary thyroid cancer when compared to placebo. Although there are differences between individual medications with respect to their effects on cardiovascular events, GLP-1 RAs offer a favorable risk-benefit profile.
Summary
The present review confirms the cardiovascular safety and efficacy vs placebo of GLP-1 RAs in patients with type 2 diabetes at moderate-to-high atherosclerotic cardiovascular risk without significant side effects. Although professional guidelines recommend metformin as the sole first-line agent, GLP-1 RAs can be used as first-line therapy in individuals with type 2 diabetes who either are intolerant to metformin or have high cardiovascular risk factors.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Global burden of diabetes. International Diabetes Federation. Diabetic atlas fifth edition 2011, Brussels. http://www.diabetesatlas.org/.
Long AN, Dagogo-Jack S. The comorbidities of diabetes and hypertension: mechanisms and approach to target organ protection. J Clin Hypertens. 2011;13(4):244–51.
American Diabetes Association. Standards of medical care in diabetes-2016, lifestyle management. Diabetes Care. 2017;40(1):S33–43.
American Diabetes Association. Standards of medical care in diabetes-2016, cardiovascular disease and risk management. Diabetes Care. 2017;40(1):S75–87.
Gourgari E, Wilhem EE, Hassanzadeh H, et al. A comprehensive review of the FDA-approved labels of diabetes drugs: indications, safety, and emerging cardiovascular safety data. J Diabetes Complicat. 2017;31:1719–27.
Cernea S, Raz I. Therapy in the early stage: incretins. Diabetes Care. 2011;34(2):S264–71.
AstraZeneca UK Limited. Byetta. Summary of product characteristics 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_duration_Product_Information/human/000698/WC500051845.pdf. Accessed 02 June 2015.
AstraZeneca UK Limited. Byetta 2015. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
Novo Nordisk Limited. Victoza. Summary of product characteristics. May 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf.
Novo Nordisk Limited. Victoza 2015. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
AstraZeneca UK Limited. Bydureon. Summary of product characteristics, June 2015. Available from URL: http://ec.europa.eu/health/documents/community-register/2011/20110617103730/anx_103730_en.pdf.
AstraZeneca UK Limited. Bydureon 2015. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
Sanofi. Lyxumia. Summary of product characteristics December 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf.
FDA approves Adlyxin Injection to treat type 2 diabetes July 27, 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208471Orig1s000Approv.pdf.
GlaxoSmithKline. Eperzan. Summary of product characteristics February 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf.
FDA approves Tanzeum to treat type 2 diabetes April 15, 2014. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm393289.htm.
Eli Lilly and Company Limited. Trulicity summary of product characteristics January 2015. https://www.medicines.org.uk/emc/medicine/29747.
Eli Lilly and Company Limited. Trulicity 2015. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails.
Manucci E, Dicembrini I, Lauria A, Pollizi P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care. 2013;36(2):S259–63.
U.S. Food and Drug Administration. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf.
•• Pfeffer MA, Claggett B, Diaz R, Dickstein K, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. ELIXA trial demonstrated that addition of lixisenatide to usual care did not significantly alter the rate of major cardiovascular events or other serious adverse events in individuals with T2DM who had had a myocardial infarction or who had been hospitalized for unstable angina within the previous 180 days.
•• Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. LEADER trial, one of the first studies of the GLP-1 RA class, demonstrated that the rate of the first occurrence of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke among individuals with T2DM was lower with liraglutide than with placebo.
•• Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. SUSTAIN-6 trial showed that semaglutide had a significant reduction of major cardiovascular events than with placebo.
•• Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. EXCSEL trial showed cardiovascular safety with exenatide extended release in individuals with T2DM and at a wide range of cardiovascular risk vs placebo.
Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
White WB, Bakris GL, Bergenstal RM, Cannon CP, Cushman WC, Fleck P, et al. Examination of cardiovascular outcomes with alogliptin versus standard of care in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162(4):620–6.
TECOS study, Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG OUTCOME investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. CANVAS program collaborative group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, et al. DEVOTE study group. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723–32.
• Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18:317–32. A review comparing GLP-1 RAs in terms of glycemic measurement, effects on weight, cardiovascular measurements, safety, and tolerability.
Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure, and body weight: systematic review and meta-analysis. BMJ Open. 2013;3:e001986.
Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily in the treatment of type 2 diabetes;: a randomized, open-label, non-inferiority study. Lancet. 2008;372:1240–50.
Blevins T, Pullman J, Malloy J, Yan P, Taylor K, Schulteis C, et al. DURATION-5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:1301–10.
Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26 week randomized, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39–47.
Buse JB, Drucker DJ, Taylor KL, Kim T, Walsh B, Hu H, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33:1255–61.
Buse JB, Sesti G, Schmidt SE, et al. Switching to once daily liraglutide from twice daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300–3.
Rosenstock J, Raccah D, Korányi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open label, active-controlled study (GetGoal-X). Diabetes Care. 2013;36:2945–51.
Dungan KM, Povedano ST, Forst T, González JGG, Atisso C, Sealls W, et al. Once weekly dulaglutide vs once daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomized, open-label, phase 3, non-inferiotity trial. Lancet. 2014;384:1349–57.
Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013;15:737–49.
Fonseca VA, Devries JH, Henry RR, et al. Reductions in systolic blood pressure with liraglutide in patients in patients with type 2 patients: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complicat. 2014;28:399–405.
Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, et al. Once weekly albiglutide versus once daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomized, open label, multicenter, non-inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2:289–97.
Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomized, open-label study. Lancet. 2013;381:117–24.
Ji L, Onishi Y, Ahn CW, Agarwal P, Chou CW, Haber H, et al. Efficacy and safety of exenatide once weekly in Asian patients with type 2 diabetes mellitus. J Diabetes Invest. 2013;4:53–61.
Primatesta P, Poulter NR. Improvement in hypertension management in England: results from the Health Survey for England 2003. J Hypertens. 2006;24:1187–92.
Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinol. 2014;155:1280–90.
Meier JJ, Rosenstock J, Hincelin-Mary A, Roy-Duval C, et al. Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized. Open-Label Trial Diabetes Care. 2015;38(7):1263–73.
• Kang YM, Jung HJ. Cardiovascular effects of glucagon-like peptide-1 receptor agonists. Endocrinol Metab. 2016;31:258–74. A detailed article analyzing the cardiovascular effects in human studies and cardiovascular outcome studies.
Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide-1 receptor are mediated through both glucagon-like peptide-1 receptor-dependent and –independent pathways. Circulation. 2008;117:2340–50.
Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide-1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–51.
Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7-36) amide an amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–6.
Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, et al. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–52.
Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiopathy. Circulation. 2004;110:955–61.
Huisamen B, Genade S, Lochner A. Signalling pathways activated by glucagon-like peptide-1 (7-36) amide in the rat heart and their role in protection against ischemia. Cardiovasc J Afr. 2008;19:77–83.
Xie Y, Wang SX, Sha WW, Zhou X, Wang WL, Han LP, et al. Effects and mechanism of glucagon-like peptide-1 on injury of rats cardiomyocytes induced by hypoxia-reoxygenation. Chin Med J. 2008;121:2134–8.
Zhao T, Parikh P, Bhashyam, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and post-ischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–13.
Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–35.
Read PA, Khan FZ, Dutka DP. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart. 2012;98:408–13.
Lonborg J, Kelbaek H, Vejlstrup M, et al. Exenatide reduces final infarct size in patient with ST-segment-elevation myocardial infarction and short duration of ischemia. Circ Cardiovasc Interv. 2012;5:288–95.
Lonborg J, Vejlstrup N, Kelbaek H. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–49.
Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim WS, et al. Cardioprotective effects of exenatide in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–60.
Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2015;170:845–54.
Nozue T, Yamada M, Tsunoda T, Katoh H, Ito S, Iwaki T, et al. Effects of liraglutide, a glucagon-like-peptide-1 analog, on ventricular remodeling assessed by cardiac magnetic resonance imaging in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessel. 2016;31(8):1239–46.
Courreges JP, Vilsboll T, Zdravkovic M, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25:1129–31.
Diaz-Soto G, de Luis DA, Conde-Vicente R, et al. Beneficial effects of liraglutide on adipocytokines, insulin sensitivity parameters, and cardiovascular risk biomarkers in patients with type 2 diabetes: a prospective study. Diabetes Res Clin Pract. 2014;104:92–6.
Gurkan E, Tarkun I, Sahin T, Cetinarslan B, Canturk Z. Evaluation of exenatide versus insulin glargine for the impact on endothelial functions and cardiovascular risk markers. Diabetes Res Clin Pract. 2014;106:567–75.
Bunck MC, Diamant M, Eliasson B, Corner A, Shaginian RM, Heine RJ, et al. Exenatide affects circulating cardiovascular risk biomarkers independently of changes in body composition. Diabetes Care. 2010;33:1734–7.
Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, et al. Interim analysis of the effects of exenatide treatment on A1c, weight, and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–47.
Rizzo M, Chandalia M, Patti AM, di Bartolo V, Rizvi AA, Montalto G, et al. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8 month prospective pilot study. Cardiovasc Diabetol. 2014;13:49.
Nezu T, Hosomi M, Aoki S, et al. Carotid intima-media thickness for atherosclerosis. J Ateroscler Thromb. 2016;23:18–31.
Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans; a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–12.
• Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6(2):105–13. A systematic review of four cardiovascular trials (ELIXA, LEADER, SUSTAIN-6, and EXSCEL) that shows cardiovascular safety and efficacy across all GLP-1 RA.
Wilcox R, Kupfer S. Erdmann E; Proactive Study investigators. Effects of pioglitazone on major adverse cardiovascular events in high-risk patients with type 2 diabetes: results from Prospective pioglitazone Clinical Trial In macro Vascular Events (PROactive 10). Am Heart J. 2008;155(4):712–7.
• Jia X, Alam M, Ye Y, et al. GLP-1 receptor agonists and cardiovascular disease: a meta-analysis of recent cardiac outcome trials. Cardiovasc Drugs Ther. 2018;32(1):65–72. A meta-analysis of ELIXA, LEADER, SUSTAIN-6, and EXSCEL trials, which demonstrate that GLP-1 RAs are cardioprotective and decrease cardiac and all-cause mortality.
AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67.
The HPS2-THRIVE collaborative group effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12.
Elam M, Lovato L, Ginsberg H. The ACCORD-lipid study: implications for treatment of dyslipidemia in type 2 diabetes mellitus. Clin Lipidol. 2011;6(1):9–20.
Faillie J-L, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L. Association of bile duct and gallbladder diseases with the use of incretin-based drugs in patients with type 2 diabetes mellitus. JAMA Intern Med. 2016;176(10):1474–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gül Bahtiyar, Jean Pujals-Kury, and Alan Sacerdote declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Bahtiyar, G., Pujals-Kury, J. & Sacerdote, A. Cardiovascular Effects of Different GLP-1 Receptor Agonists in Patients with Type 2 Diabetes. Curr Diab Rep 18, 92 (2018). https://doi.org/10.1007/s11892-018-1043-z
Published:
DOI: https://doi.org/10.1007/s11892-018-1043-z