Abstract
Purpose of Review
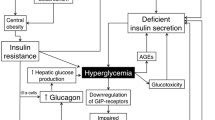
Hyperglucagonemia contributes significantly to hyperglycemia in type 2 diabetes and suppressed glucagon levels may increase the risk of hypoglycemia. Here, we give a brief overview of glucagon physiology and the role of glucagon in the pathophysiology of type 2 diabetes and provide insights into how antidiabetic drugs influence glucagon secretion as well as a perspective on the future of glucagon-targeting drugs.
Recent Findings
Several older as well as recent investigations have evaluated the effect of antidiabetic agents on glucagon secretion to understand how glucagon may be involved in the drugs’ efficacy and safety profiles. Based on these findings, modulation of glucagon secretion seems to play a hitherto underestimated role in the efficacy and safety of several glucose-lowering drugs.
Summary
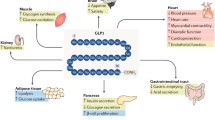
Numerous drugs currently available to diabetologists are capable of altering glucagon secretion: metformin, sulfonylurea compounds, insulin, glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, sodium-glucose cotransporter 2 inhibitors and amylin mimetics. Their diverse effects on glucagon secretion are of importance for their individual efficacy and safety profiles. Understanding how these drugs interact with glucagon secretion may help to optimize treatment.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Can Med Assoc J. 1922;12:141–6.
Murlin JR, Clough HD, Gibbs CBF, Stokes AM. Aqueous extracts of pancreas I. Influence on the carbohydrate metabolism of depancreatized animals. J Biol Chem. 1923;56:253–96.
• Lund A, Bagger JI, Christensen M, Knop FK, Vilsbøll T. Glucagon and type 2 diabetes: the return of the alpha cell. Curr Diab Rep. 2014;14:1–7. This paper excellently summarizes the importance of glucagon in type 2 diabetes.
Nauck MA, Vilsbøll T, Gallwitz B, Garber A, Madsbad S. Incretin-based therapies. Diabetes Care. 2009;32:S223–31.
Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95:513–48.
Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186:629–37.
Boron, W. F. & Boulpaep, E. L. Medical Physiology: A cellular and molecular approach. Elsevier Health Sciences, 2012, 2nd edition, ISBN 978-0-8089-2449-4.
Habegger KM, et al. The metabolic actions of glucagon revisited. Nat. Rev. Endocrinol. 2010;6:689–97.
Charron MJ, Vuguin PM. Lack of glucagon receptor signaling and its implications beyond glucose homeostasis. J Endocrinol. 2015;224:R123–30.
Eaton RP. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. J Lipid Res. 1973;14:312–8.
Billington CJ, Briggs JE, Link JG, Levine AS. Glucagon in physiological concentrations stimulates brown fat thermogenesis in vivo. Am J Phys. 1991;261:R501–7.
Doi K, Kuroshima A. Modified metabolic responsiveness to glucagon in cold-acclimated and heat-acclimated rats. Life Sci. 1982;30:785–91.
Inokuchi A, Oomura Y, Nishimura H. Effect of intracerebroventricularly infused glucagon on feeding behavior. Physiol Behav. 1984;33:397–400.
Ceriello, A., Genovese, S., Mannucci, E. & Gronda, E. Glucagon and heart in type 2 diabetes: new perspectives. Cardiovasc. Diabetol. 2016;15:123.
• Unger R, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975;305:14–6. The original bi-hormonal hypothesis, stated by Unger and his group.
Holst JJ, et al. Regulation of glucagon secretion by incretins. Diabetes Obes Metab. 2011;13:89–94.
Gromada J, Franklin I, Wollheim CB. α-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116.
Reaven GM, Chen Y-DI, Golay A, Swislocki ALM, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64:106–10.
• Knop FK, Vilsbøll T, Madsbad S, Holst JJ, Krarup T. Inappropriate suppression of glucagon during OGTT but not during isoglycaemic i.v. glucose infusion contributes to the reduced incretin effect in type 2 diabetes mellitus. Diabetologia. 2007;50:797–805. This study demonstrates the inability of patients with type 2 diabetes to correctly suppress glucagon levels after oral intake of glucose.
Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–83.
Shah P, et al. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4053–9.
Dinneen S, Alzaid A, Turk D, Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia. 1995;38:337–43.
• Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–7. The highly debated study, in which glucagon action is essential to the development of diabetes.
Lee Y, et al. Metabolic manifestations of insulin deficiency do not occur without glucagon action. Proc Natl Acad Sci U S A. 2012;109:14972–6.
Mani BK, et al. Hypoglycemic effect of combined ghrelin and glucagon receptor blockade. Diabetes. 2017;66:1847–57.
Steenberg, V. R. et al. Acute disruption of glucagon secretion or action does not improve glucose tolerance in an insulin-deficient mouse model of diabetes. Diabetol. Heidelb. 2016;59:363–370.
Damond N, et al. Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist. Elife. 2016;5:e13828.
Holst JJ, et al. Insulin and glucagon: partners for life. Endocrinology. 2017;158:696–701.
Knop FK, et al. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab. 2012;14:500–10.
Kelly RP, et al. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy people and in those with type 2 diabetes. Diabetes Obes Metab. 2015;17:414–22.
Muscelli E, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–8.
• Lund A, et al. Evidence of extrapancreatic glucagon secretion in man. Diabetes. 2016;65:585–97. This study shows that glucagon can be produced in fully pancreatectomized patients, possibly from the gut.
Bagger JI, et al. Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96:737–45.
Christensen MB, Calanna S, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99:E418–26.
Chia CW, et al. Exogenous glucose–dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes. 2009;58:1342–9.
Lund A, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. Am J Physiol - Endocrinol Metab. 2011;300:E1038–46.
MacDonald, P. E. et al. A KATP Channel-Dependent Pathway within α Cells Regulates Glucagon Release from Both Rodent and Human Islets of Langerhans. PLOS Biol. 2007;5:e143.
Jin T. Mechanisms underlying proglucagon gene expression. J Endocrinol. 2008;198:17–28.
De Marinis YZ, et al. GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab. 2010;11:543–53.
Rorsman P, Braun M, Zhang Q. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium. 2012;51:300–8.
McCulloch DK, Raghu PK, Koerker DJ, Palmer JP, Klaff LJ. Responses of the pancreatic A cell during hypoglycemia and hyperglycemia are dependent on the B cell. Metabolism. 1989;38:702–7.
Rocha DM, Faloona GR, Unger RH. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972;51:2346–51.
Meier DJJ, et al. Gastric inhibitory polypeptide (GIP) dose-dependently stimulates glucagon secretion in healthy human subjects at euglycaemia. Diabetologia. 2003;46:798–801.
Meier JJ, et al. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130:44–54.
Ahrén B, Veith RC, Taborsky GJ. Sympathetic nerve stimulation versus pancreatic norepinephrine infusion in the dog: 1. Effects on basal release of insulin and glucagon. Endocrinology. 1987;121:323–31.
Ahrén B, Taborsky GJ. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology. 1986;118:1551–7.
Dunning BE, Foley JE, Ahrén B. Alpha cell function in health and disease: influence of glucagon-like peptide-1. Diabetologia. 2005;48:1700–13.
Asmar M, Bache M, Knop FK, Madsbad S, Holst JJ. Do the actions of glucagon-like peptide-1 on gastric emptying, appetite, and food intake involve release of amylin in humans? J Clin Endocrinol Metab. 2010;95:2367–75.
Gedulin BR, Jodka CM, Herrmann K, Young AA. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul Pept. 2006;137:121–7.
Gedulin BR, Rink TJ, Young AA. Dose-response for glucagonostatic effect of amylin in rats. Metabolism. 1997;46:67–70.
de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia. 2008;51:2263–70.
Zhang Q, et al. Role of KATP channels in glucose-regulated glucagon secretion and impaired Counterregulation in type 2 diabetes. Cell Metab. 2013;18:871–82.
Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB. β-cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes. 2005;54:1808–15.
Xu E, et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell Metab. 2006;3:47–58.
Sun EW, et al. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes. 2017;66:2144–9.
Kiec-Klimczak ME, Pach DM, Pogwizd ME, Hubalewska-Dydejczyk AB. Incretins yesterday, pleiotropic gastrointestinal hormones today: glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Recent Pat Endocr Metab Immune Drug Discov. 2011;5:176–82.
Ørgaard A, Holst JJ. The role of somatostatin in GLP-1-induced inhibition of glucagon secretion in mice. Diabetologia. 2017:1–9. https://doi.org/10.1007/s00125-017-4315-2.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Hartmann B, et al. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides. 2000;21:73–80.
de Heer J, Pedersen J, Ørskov C, Holst JJ. The alpha cell expresses glucagon-like peptide-2 receptors and glucagon-like peptide-2 stimulates glucagon secretion from the rat pancreas. Diabetologia. 2007;50:2135–42.
Wasserman DH. Four grams of glucose. Am. J. Physiol. - Endocrinol Metab. 2009;296:E11–21.
Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167.
Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–14.
Pernicova I, Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014;10:143–56.
Umpierrez G, Povedano ST, Manghi FP, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37:2168–76.
Bi Y, et al. The beneficial effect of metformin on β-cell function in non-obese Chinese subjects with newly diagnosed type 2 diabetes. Diabetes Metab Res Rev. 2013;29:664–72.
Machado HA, et al. Metformin, but not glimepiride, improves carotid artery diameter and blood flow in patients with type 2 diabetes mellitus. Clinics. 2012;67:711.
Vardarli I, Arndt E, Deacon CF, Holst JJ, Nauck MA. Effects of sitagliptin and metformin treatment on incretin hormone and insulin secretory responses to oral and ‘Isoglycemic’ intravenous glucose. Diabetes. 2014;63:663–74.
Koster JC, Permutt MA, Nichols CG. Diabetes and insulin secretion. Diabetes. 2005;54:3065–72.
Ashcroft, F. M. & Rorsman, P. ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion. Biochem Soc Trans. 18, 109–111 (1990).
Association, A. D. 8. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40:S64–74.
Thulé PM, Umpierrez G. Sulfonylureas: a new look at old therapy. Curr. Diab. Rep. 2014;14:473.
Landstedt-Hallin L, Adamson U, Lins P-E. Oral glibenclamide suppresses glucagon secretion during insulin-induced hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab. 1999;84:3140–5.
Evans ML, et al. Hypothalamic ATP-sensitive K + channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes. 2004;53:2542–51.
Joy NG, Tate DB, Davis SN. Counterregulatory responses to hypoglycemia differ between glimepiride and glyburide in non diabetic individuals. Metabolism. 2015;64:729–37.
Szoke E, et al. Effects of glimepiride and glyburide on glucose counterregulation and recovery from hypoglycemia. Metabolism. 2006;55:78–83.
Ahrén B, et al. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–84.
Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011;13:7–18.
Kim D, et al. (2R)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin- 7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48:141–51.
Yabe D, et al. Effects of DPP-4 inhibitor linagliptin and GLP-1 receptor agonist liraglutide on physiological response to hypoglycaemia in Japanese subjects with type 2 diabetes: a randomized, open-label, 2-arm parallel comparative, exploratory trial. Diabetes Obes Metab. 2017;19:442–7.
Ahrén B, et al. Vildagliptin enhances islet responsiveness to both hyper- and hypoglycemia in patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1236–43.
Drucker DJ, et al. Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nat Biotechnol. 1997;15:673–7.
Aaboe K, et al. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:323–33.
•• Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18:203–16. This review gives a sound overview of incretin therapy.
A/S, N. N. Novo Nordisk to initiate phase 3a development of oral semaglutide, a once-daily oral GLP-1 analogue. GlobeNewswire News Room (2015). Available at: http://globenewswire.com/news-release/2015/08/26/763616/0/en/Novo-Nordisk-to-initiate-phase-3a-development-of-oral-semaglutide-a-once-daily-oral-GLP-1-analogue.html. (Accessed: 31st July 2017).
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet Lond. Engl. 2006;368:1696–705.
Dupré J, Behme MT, McDonald TJ. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J Clin Endocrinol Metab. 2004;89:3469–73.
Retnakaran R, Kramer CK, Choi H, Swaminathan B, Zinman B. Liraglutide and the preservation of pancreatic β-cell function in early type 2 diabetes: the LIBRA trial. Diabetes Care. 2014;37:3270–8.
Buse JB, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet Lond Engl. 2013;381:117–24.
Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care. 2013;36:2543–50.
Rosenstock J, et al. Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). J Diabetes Complicat. 2014;28:386–92.
Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight from the LIBRA trial. J Clin Endocrinol Metab. 2015;100:3702–9.
Kalra, S. & Gupta, Y. Letter to the editor: comment on ‘The Impact of Chronic Liraglutide Therapy on Glucagon Secretion in Type 2 Diabetes: Insight From the LIBRA Trial’ by Kramer C.K, et al. J Clin Endocrinol Metab. 100, L116–L117 (2015).
•• Hare KJ, et al. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2010;59:1765–70. This study attributes GLP-1’s glucagonostatic effect 50% of its glucose-lowering effect.
Storgaard H, et al. Benefits and harms of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2016;11:e0166125.
Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502.
Merovci A, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–14.
Ferrannini E, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:1868.
Bonner C, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–7.
Association, A. D. Integrated physiology/obesity. Diabetes. 2017;66:A496–565.
Goldenberg RM, Verma S, Perkins BA, Gilbert JD, Zinman B. Can the combination of incretin agents and sodium-glucose cotransporter 2 (SGLT2) inhibitors reconcile the yin and yang of glucagon? Can. J Diabetes. 2017;41:6–9.
Mathieu C, et al. Randomized, double-blind, phase 3 trial of triple therapy with dapagliflozin add-on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009–17.
Mathieu C, et al. Efficacy and safety of triple therapy with dapagliflozin add-on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:1134–7.
Matthaei S, et al. Randomized, double-blind trial of triple therapy with saxagliptin add-on to dapagliflozin plus metformin in patients with type 2 diabetes. Diabetes Care. 2015;38:2018–24.
Rosenstock J, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38:376–83.
DeFronzo RA, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384–93.
Lewin A, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care. 2015;38:394–402.
Hansen L, Iqbal N, Ekholm E, Cook W, Hirshberg B. Postprandial dynamics of plasma glucose, insulin, and glucagon in patients with type 2 diabetes treated with saxagliptin plus dapagliflozin add-on to metformin therapy. Endocr. Pract. Jacksonv. 2014;20:1187–97.
Frías JP, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–16.
Fulcher G, et al. Efficacy and safety of canagliflozin when used in conjunction with incretin-mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:82–91.
Saroka RM, Kane MP, Busch RS, Watsky J, Hamilton RA. Sglt-2 inhibitor therapy added to Glp-1 agonist therapy in the management of T2dm. Endocr Pract Jacksonv. 2015;21:1315–22.
Raskin P, et al. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28:260–5.
Raskin P, Fujita Y, Unger RH. Effect of insulin-glucose infusions on plasma glucagon levels in fasting diabetics and nondiabetics. J Clin Invest. 1975;56:1132.
Raskin P, Unger RH. Effect of insulin therapy on the profiles of plasma immunoreactive glucagon in juvenile-type and adult-type diabetics. Diabetes. 1978;27:411–9.
Ohneda A, Ishii S, Horigome K, Yamagata S. Glucagon response to arginine after treatment of diabetes mellitus. Diabetes. 1975;24:811–9.
He, Y., Wu, B., Meng, M., Ma, W. & Zhu, D. Effects of insulin therapy on glucagon in patients with newly diagnosed type 2 diabetes. Biomed Res. Allied Academics, sept 28 (2017).
Launay B, Zinman B, Tildesley HD, Strack T, Chiasson JL. Effect of continuous subcutaneous insulin infusion with lispro on hepatic responsiveness to glucagon in type 1 diabetes. Diabetes Care. 1998;21:1627–31.
Jorsal T, Rungby J, Knop FK, Vilsbøll T. GLP-1 and amylin in the treatment of obesity. Curr Diab Rep. 2016;16:1.
Fineman M, Weyer C, Maggs DG, Strobel S, Kolterman OG. The human amylin analog, pramlintide, reduces postprandial hyperglucagonemia in patients with type 2 diabetes mellitus. Horm Metab Res. 2002;34:504–8.
Levetan C, et al. Impact of pramlintide on glucose fluctuations and postprandial glucose, glucagon, and triglyceride excursions among patients with type 1 diabetes intensively treated with insulin pumps. Diabetes Care. 2003;26:1–8.
Hollander PA, Levy P, Fineman MS, Maggs DG, et al. Pramlintide as an adjunct to insulin therapy improves long-term glycemic and weight control in patients with type 2 diabetes: a 1-year randomized controlled trial. Diabetes Care Alex. 2003;26:784–90.
Young A. Inhibition of glucagon secretion. Adv Pharmacol. 2005;52:151–71.
Bagger JI, Knop FK, Holst JJ, Vilsbøll T. Glucagon antagonism as a potential therapeutic target in type 2 diabetes. Diabetes Obes Metab. 2011;13:965–71.
• Kazda CM, et al. Evaluation of efficacy and safety of the glucagon receptor antagonist LY2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care. 2016;39:1241–9. This study highlights the potential of future antagonism of the glucagon receptor.
Mu J, et al. Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice. Diabetologia. 2011;54:2381–91.
Pocai A, et al. Glucagon-like peptide 1/glucagon receptor dual Agonism reverses obesity in mice. Diabetes. 2009;58:2258–66.
Finan, B., Clemmensen, C. & Müller, T. D. Emerging opportunities for the treatment of metabolic diseases: glucagon-like peptide-1 based multi-agonists. Mol Cell Endocrinol. 418, 42–54 (2015).
Soni H. Peptide-based GLP-1/glucagon co-agonists: a double-edged sword to combat diabesity. Med Hypotheses. 2016;95:5–9.
Finan B, et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat Med. 2015;21:27–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Magnus F. Grøndahl is a minority shareholder in the Danish biotech company Zealand Pharma; Damien J. Keating has no conflict of interest. Tina Vilsbøll has received lecture fees from, participated in advisory boards of, consulted for and/or received research grants from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, MSD/Merck, Novo Nordisk, and Sanofi. Filip K. Knop has received lecture fees from, participated in advisory boards of, consulted for and/or received research grants from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD/Merck, Novo Nordisk, Sanofi, and Zealand Pharma.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Grøndahl, M.F., Keating, D.J., Vilsbøll, T. et al. Current Therapies That Modify Glucagon Secretion: What Is the Therapeutic Effect of Such Modifications?. Curr Diab Rep 17, 128 (2017). https://doi.org/10.1007/s11892-017-0967-z
Published:
DOI: https://doi.org/10.1007/s11892-017-0967-z