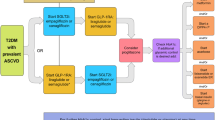
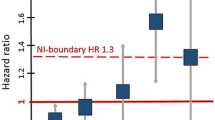
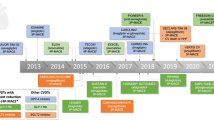
Abstract
Cardiovascular disease (CVD) is common in patients with diabetes. For these patients, clinicians should seek diabetes treatment that is beneficial rather than harmful in relation to CVD. Until recently, there have been many treatments for hyperglycemia, whose impact on CVD has been controversial. The aims of this review are to evaluate the effectiveness of antihyperglycemic medications on risk factors for CVD and to examine the impact of these drugs on CVD in cardiovascular (CV) outcome trials. In this article, we summarize current knowledge about the impacts of these drugs on various risk factors as well as CV outcomes. We identify the recent emergence of trials with antihyperglycemic agents showing newly discovered CV benefits as well as past trials with antihyperglycemic agents not showing much benefit on CV events. Rather than focusing on treatment strategies, we review the effects of individual drug classes on CV outcomes. We also briefly review goal-driven glycemia reduction and its impact on CVD. We conclude that antihyperglycemic agents are associated with improvement in CV risk factors in patients with diabetes and insulin resistance; in fact, a few drugs reduced CV events in randomized CV outcome trials. Therefore, the use of these drugs is appropriate for reducing glucose and decreasing CV event risk in a select subpopulation.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–46.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Jama. 2003;289:2560–71.
Bauters C, Lamblin N, Mc Fadden EP, Van Belle E, Millaire A, de Groote P. Influence of diabetes mellitus on heart failure risk and outcome. Cardiovasc Diabetol. 2003;2:1.
Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff Jr DC, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–28.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39.
Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–206.
Cushman WC, Evans GW, Byington RP, Goff Jr DC, Grimm Jr RH, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. In N Engl J Med. 2010;362:1575–85. Edited by: 2010 Massachusetts Medical Society.
Ginsberg HN, Elam MB, Lovato LC, Crouse 3rd JR, Leiter LA, Linz P, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. In N Engl J Med. 2010;362:1563–74. Edited by: 2010 Massachusetts Medical Society.
Del Prato S, LaSalle J, Matthaei S, Bailey CJ. Tailoring treatment to the individual in type 2 diabetes practical guidance from the Global Partnership for Effective Diabetes Management. Int J Clin Pract. 2010;64:295–304.
Hirshberg B, Raz I. Impact of the U.S. Food and Drug Administration cardiovascular assessment requirements on the development of novel antidiabetes drugs. Diabetes Care. 2011;34 Suppl 2:S101–106.
Gubitosi-Klug RA. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: summary and future directions. Diabetes Care. 2014;37:44–9.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:854–65.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:837–53.
Kelly TN, Bazzano LA, Fonseca VA, Thethi TK, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. This study as well as the LEADER study showed a newly recognized CV benefit of antihyperglycemic agents and according to experts in the field this discovery may lead to empagliflozin or liraglutide use as a first-line therapy along with metformin in patients with T2DM and atherosclerotic CVD.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31. This study was important as it showed a new approach to stroke prevention using an insulin-sensitizing agent in non-diabetic participant with insulin resistance. This study paves the way for futures studies in the area of insulin resistance before development of diabetes.
Kooy A, de Jager J, Lehert P, Bets D, Wulffele MG, Donker AJ, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–25.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89.
Chiasson JL, Gomis R, Hanefeld M, Josse RG, Karasik A, Laakso M. The STOP-NIDDM Trial: an international study on the efficacy of an alpha-glucosidase inhibitor to prevent type 2 diabetes in a population with impaired glucose tolerance: rationale, design, and preliminary screening data. Study to prevent non-insulin-dependent diabetes mellitus. Diabetes Care. 1998;21:1720–5.
Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. Jama. 2003;290:486–94.
Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc. 2012;1:e002279.
Cycloset FDA approved Package Insert. Patheon Inc., 4/2009;1-14. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020866lbl.pdf. Accessed 12 Aug 2016.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71.
Services USDoHaH, Administration FaD, (CDER) CfDEaR. Guidance for industry diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Center for Drug Evaluation and Research Food and Drug Administration, editor. 2008.
Hirshberg B, Katz A. Cardiovascular outcome studies with novel antidiabetes agents: scientific and operational considerations. Diabetes Care. 2013;36 Suppl 2:S253–8.
Gautier JF, Fetita S, Sobngwi E, Salaun-Martin C. Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes. Diabetes Metab. 2005;31:233–42.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. This study as well as the EMPA-REG OUTCOME study showed a newly recognized CV benefit of antihyperglycemic agents, and according to experts in the field, this discovery may lead to empagliflozin or liraglutide use as a first-line therapy along with metformin in patients with T2DM and atherosclerotic CVD.
Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19–28.
Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37:2168–76.
Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880–6.
Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–93.
Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–86.
Ceriello A, Novials A, Ortega E, Canivell S, La Sala L, Pujadas G, et al. Glucagon-like peptide 1 reduces endothelial dysfunction, inflammation, and oxidative stress induced by both hyperglycemia and hypoglycemia in type 1 diabetes. Diabetes Care. 2013;36:2346–50.
Garber AJ. Incretin effects on beta-cell function, replication, and mass: the human perspective. Diabetes Care. 2011;34 Suppl 2:S258–63.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57.
Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–35.
Reimer MK, Holst JJ, Ahren B. Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur J Endocrinol. 2002;146:717–27.
Rosenstock J, Inzucchi SE, Seufert J, Fleck PR, Wilson CA, Mekki Q. Initial combination therapy with alogliptin and pioglitazone in drug-naive patients with type 2 diabetes. Diabetes Care. 2010;33:2406–8.
DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315–7.
Meneghini LF, Orozco-Beltran D, Khunti K, Caputo S, Damci T, Liebl A, et al. Weight beneficial treatments for type 2 diabetes. J Clin Endocrinol Metab. 2011;96:3337–53.
Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis. 2013;226:305–14.
Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K, et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77:1337–44.
Noda Y, Miyoshi T, Oe H, Ohno Y, Nakamura K, Toh N, et al. Alogliptin ameliorates postprandial lipemia and postprandial endothelial dysfunction in non-diabetic subjects: a preliminary report. Cardiovasc Diabetol. 2013;12:8.
Monami M, Vitale V, Ambrosio ML, Bartoli N, Toffanello G, Ragghianti B, et al. Effects on lipid profile of dipeptidyl peptidase 4 inhibitors, pioglitazone, acarbose, and sulfonylureas: meta-analysis of placebo-controlled trials. Adv Ther. 2012;29:736–46.
van Genugten RE, Moller-Goede DL, van Raalte DH, Diamant M. Extra-pancreatic effects of incretin-based therapies: potential benefit for cardiovascular-risk management in type 2 diabetes. Diabetes Obes Metab. 2013;15:593–606.
Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, Steg PG, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 Trial. Diabetes Care. 2015;38:696–705.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36:3460–8.
Schernthaner G, Sattar N. Lessons from SAVOR and EXAMINE: some important answers, but many open questions. J Diabetes Complications. 2014;28:430–3.
Cavender MA, Scirica BM, Raz I, Gabriel Steg P, McGuire DK, Leiter LA, et al. Cardiovascular outcomes of patients in SAVOR-TIMI 53 by baseline hemoglobin A1c. Am J Med. 2016;129:340. e341-348.
Zannad F, Cannon CP, Cushman WC, Bakris GL, Menon V, Perez AT, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067–76.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
Green JB, Bethel MA, Paul SK, Ring A, Kaufman KD, Shapiro DR, et al. Rationale, design, and organization of a randomized, controlled trial evaluating cardiovascular outcomes with sitagliptin (TECOS) in patients with type 2 diabetes and established cardiovascular disease. Am Heart J. 2013;166:983–9. e987.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.
Marx N, Rosenstock J, Kahn SE, Zinman B, Kastelein JJ, Lachin JM, et al. Design and baseline characteristics of the Cardiovascular Outcome Trial of Linagliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA(R)). Diab Vasc Dis Res. 2015;12:164–74.
Petersen C. Analyse des phloridzins. Annalen der Pharmacie. 1835;15:178.
Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–8.
FDA. FDA approves invokana to treat type 2 diabetes. In: FDA, editor. First in a new class of diabetes drugs. 2013.
Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22:753–62.
Hall JE. Guyton and Hall textbook of medical physiology. Philadelphia: Elsevier Health Sciences; 2015.
Komoroski B, Vachharajani N, Boulton D, Kornhauser D, Geraldes M, Li L, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–6.
Sarashina A, Koiwai K, Seman LJ, Yamamura N, Taniguchi A, Negishi T, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet. 2013;28:213–9.
Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–72.
Oliva RV, Bakris GL. Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens. 2014;8:330–9.
Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–74.
Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2015;309:F889–900.
Zinman B, Inzucchi SE, Lachin JM, Wanner C, Ferrari R, Fitchett D, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME). Cardiovasc Diabetol. 2014;13:102.
Janssen Research & Development, LLC. CANagliflozin cardioVascular Assessment Study (CANVAS). 2016. https://clinicaltrials.gov/ct2/show/NCT01032629?term=NCT01032629&rank=1studyidentifier:NCT01032629. Accessed 22 Mar 2016
AstraZeneca. Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58). 2016. https://clinicaltrials.gov/ct2/show/NCT01730534. Accessed 23 Mar 2016
Hanefeld M, Marx N, Pfutzner A, Baurecht W, Lubben G, Karagiannis E, et al. Antiinflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: the PIOSTAT Study. J Am Coll Cardiol 2007;49:290–297
Bakris GL, Ruilope LM, McMorn SO, Weston WM, Heise MA, Freed MI, et al. Rosiglitazone reduces microalbuminuria and blood pressure independently of glycemia in type 2 diabetes patients with microalbuminuria. J Hypertens 2006;24:2047–55
Gilling L, Suwattee P, DeSouza C, Asnani S, Fonseca V. Effects of the thiazolidinediones on cardiovascular risk factors. Am J Cardiovasc Drugs 2002;2:149–156
Fonseca VA. Rationale for the use of insulin sensitizers to prevent cardiovascular events in type 2 diabetes mellitus. Am J Med 2007;120:S18–25
Fonseca V, McDuffie R, Calles J, Cohen RM, Feeney P, Feinglos M, et al. Determinants of weight gain in the action to control cardiovascular risk in diabetes trial. Diabetes Care 2013;36:2162–68
Erdmann E, Song E, Spanheimer R, van Troostenburg de Bruyn AR, Perez A. Observational follow-up of the PROactive study: a 6-year update. Diabetes Obes Metab 2014;16:63–74
Acknowledgments
Dr. Fonseca is supported in part by the Tullis Tulane Alumni Chair in Diabetes, the Patient Centered Outcomes Research Institute (through LACDRN), and grant 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
All authors contributed to the literature search and writing of the manuscript. V.F. designed the search strategy, supervised study implementation, and reviewed/edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Vivian A. Fonseca reports grants from Eli Lilly, Abbott, Mesoblast, Asahi, Bristol-Myers Squibb, Gilead. He reports others from Takeda, Sanofi-Aventis, Intarcia, and Novo Nordisk. He reports personal fees from Intarcia, Merck, GlaxoSmithKline, Sanofi-Aventis, Eli Lilly, Astra-Zeneca, Pan American Laboratories, Takeda, Jansen, Boehringer Ingelheim, and NuMe Health/Microbiome.
Dragana Lovre, Wynn Htun, and Carly Carrion declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Lovre, D., Htun, W., Carrion, C. et al. What Are We Learning from the FDA-Mandated Cardiovascular Outcome Studies for New Pharmacological Antidiabetic Agents?. Curr Diab Rep 16, 94 (2016). https://doi.org/10.1007/s11892-016-0788-5
Published:
DOI: https://doi.org/10.1007/s11892-016-0788-5