Abstract
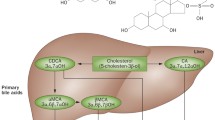
Bile acids are synthesized in the liver from cholesterol and have traditionally been recognized for their role in absorption of lipids and in cholesterol homeostasis. In recent years, however, bile acids have emerged as metabolic signaling molecules that are involved in the regulation of lipid and glucose metabolism, and possibly energy homeostasis, through activation of the bile acid receptors farnesoid X receptor (FXR) and TGR5. Bile acid sequestrants (BASs) constitute a class of drugs that bind bile acids in the intestine to form a nonabsorbable complex resulting in interruption of the enterohepatic circulation. This increases bile acid synthesis and consequently reduces serum low-density lipoprotein cholesterol. Also, BASs improve glycemic control in patients with type 2 diabetes. Despite a growing understanding of the impact of BASs on glucose metabolism, the mechanisms behind their glucose-lowering effect in patients with type 2 diabetes remain unclear. This article offers a review of the mechanisms behind the glucose-lowering effect of BASs, and the efficacy of BASs in the treatment of type 2 diabetes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91. doi:10.1152/physrev.00010.2008.
Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi:10.1074/jbc.M209706200.
Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–90. doi:10.1016/j.bbrc.2005.01.139.
Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77. doi:10.1016/j.cmet.2009.08.001.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi:10.1038/nature04330.
Out C, Groen AK, Brufau G. Bile acid sequestrants: more than simple resins. Curr Opin Lipidol. 2012;23:43–55. doi:10.1097/MOL.0b013e32834f0ef3.
Porez G, Prawitt J, Gross B, Staels B. Bile acid receptors as targets for the treatment of dyslipidemia and cardiovascular disease. J Lipid Res. 2012;53:1723–37. doi:10.1194/jlr.R024794.
Rosenbaum DP, Petersen JS, Ducharme S, et al. Absorption, distribution and excretion of GT31-104, a novel bile acid sequestrant, in rats and dogs after acute and subchronic administration. J Pharm Sci. 1997;86:591–5. doi:10.1021/js9603820.
Heller DP, Burke SK, Davidson DM, Donovan JM. Absorption of colesevelam hydrochloride in healthy volunteers. Ann Pharmacother. 2002;36:398–403.
Davidson MH, Dillon MA, Gordon B, et al. Colesevelam hydrochloride (cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med. 1999;159:1893–900.
Lyons D, Webster J, Fowler G, Petrie JC. Colestipol at varying dosage intervals in the treatment of moderate hypercholesterolaemia. Br J Clin Pharmacol. 1994;37:59–62.
Zieve FJ, Kalin MF, Schwartz SL, et al. Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29:74–83. doi:10.1016/j.clinthera.2007.01.003.
The Lipid Research Clinics Coronary Primary Prevention Trial results. II The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–74.
Garg A, Grundy SM. Cholestyramine therapy for dyslipidemia in noninsulin-dependent diabetes mellitus. A short-term, double-blind, crossover trial. Ann Intern Med. 1994;121:416–22.
Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168:1975–83. doi:10.1001/archinte.168.18.1975.
Fonseca VA, Rosenstock J, Wang AC, et al. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479–84. doi:10.2337/dc08-0283.
Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531–40. doi:10.1001/archinte.168.14.1531.
Neda T, Inukai K, Kurihara S, et al. Hypoglycemic effects of colestimide on type 2 diabetic patients with obesity. Endocr J. 2012;59:239–46.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327–36.
Braunlin W, Zhorov E, Smisek D, et al. In vitro comparison of bile acid binding to colesevelam HCl and other bile acid sequestrants. Polym Prepr. 2000;41:708–9.
Steinmetz KL. Colesevelam hydrochloride. Am J Health Syst Pharm. 2002;59:932–9.
Di Angelantonio E, Sarwar N, et al. Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi:10.1001/jama.2009.1619.
Donovan JM, Stypinski D, Stiles MR, et al. Drug interactions with colesevelam hydrochloride, a novel, potent lipid-lowering agent. Cardiovasc Drugs Ther. 2000;14:681–90.
Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5.
Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8.
Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–53.
Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–71. doi:10.1152/physrev.00027.2002.
Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. doi:10.1016/j.cmet.2005.09.001.
Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26.
Keitel V, Ullmer C, Häussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391:785–9. doi:10.1515/BC.2010.077.
Keitel V, Donner M, Winandy S, et al. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi:10.1016/j.bbrc.2008.04.171.
Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi:10.1002/hep.21458.
Potthoff MJ, Potts A, He T, et al. Colesevelam suppresses hepatic glycogenolysis by TGR5-mediated induction of GLP-1 action in DIO mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G371–80. doi:10.1152/ajpgi.00400.2012. This study establishes the concept that bile acids bound to a BAS can activate TGR5.
Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298:714–9.
Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22(814–25):e227–8. doi:10.1111/j.1365-2982.2010.01487.x.
Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–54. doi:10.1053/j.gastro.2012.09.055.
Kumar DP, Rajagopal S, Mahavadi S, et al. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem Biophys Res Commun. 2012;427:600–5. doi:10.1016/j.bbrc.2012.09.104.
Garbutt JT, Kenney TJ. Effect of cholestyramine on bile acid metabolism in normal man. J Clin Invest. 1972;51:2781–9. doi:10.1172/JCI107100.
Angelin B, Björkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982;70:724–31.
Brufau G, Stellaard F, Prado K, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52:1455–64. doi:10.1002/hep.23831.
Brufau G, Bahr MJ, Staels B, et al. Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab. 2010;7:73. doi:10.1186/1743-7075-7-73.
Meissner M, Herrema H, van Dijk TH, et al. Bile acid sequestration reduces plasma glucose levels in db/db mice by increasing its metabolic clearance rate. PLoS ONE. 2011;6:e24564. doi:10.1371/journal.pone.0024564.
Sugimoto-Kawabata K, Shimada H, Sakai K, et al. Colestilan decreases weight gain by enhanced NEFA incorporation in biliary lipids and fecal lipid excretion. J Lipid Res. 2013;54:1255–64. doi:10.1194/jlr.M032839.
Henry RR, Aroda VR, Mudaliar S, et al. Effects of colesevelam on glucose absorption and hepatic/peripheral insulin sensitivity in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:40–6. doi:10.1111/j.1463-1326.2011.01486.x.
Schwartz SL, Lai Y-L, Xu J, et al. The effect of colesevelam hydrochloride on insulin sensitivity and secretion in patients with type 2 diabetes: a pilot study. Metab Syndr Relat Disord. 2010;8:179–88. doi:10.1089/met.2009.0049.
Smushkin G, Sathananthan M, Piccinini F, et al. The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes. 2013;62:1094–101. doi:10.2337/db12-0923. This study suggests that BASs may decrease meal appearance rate.
Beysen C, Murphy EJ, Deines K, et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomized controlled study. Diabetologia. 2012;55:432–42. doi:10.1007/s00125-011-2382-3.
Adrian TE, Gariballa S, Parekh KA, et al. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia. 2012;55:2343–7. doi:10.1007/s00125-012-2593-2.
Shang Q, Saumoy M, Holst JJ, et al. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419–24. doi:10.1152/ajpgi.00362.2009.
Shang Q, Liu MK, Saumoy M, et al. The combination of colesevelam with sitagliptin enhances glycemic control in diabetic ZDF rat model. Am J Physiol Gastrointest Liver Physiol. 2012;302:G815–23. doi:10.1152/ajpgi.00295.2011.
Chen L, McNulty J, Anderson D, et al. Cholestyramine reverses hyperglycemia and enhances glucose-stimulated glucagon-like peptide 1 release in Zucker diabetic fatty rats. J Pharmacol Exp Ther. 2010;334:164–70. doi:10.1124/jpet.110.166892.
Harach T, Pols TWH, Nomura M, et al. TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep. 2012;2:430. doi:10.1038/srep00430. This study show that BASs promote GLP-1 secretion in a TGR5-dependent manner, and that the colon is the major source of the enhanced GLP-1 secretion.
Suzuki T, Oba K, Igari Y, et al. Colestimide lowers plasma glucose levels and increases plasma glucagon-like PEPTIDE-1 (7-36) levels in patients with type 2 diabetes mellitus complicated by hypercholesterolemia. J Nihon Med Sch. 2007;74:338–43.
Garg SK, Ritchie PJ, Moser EG, et al. Effects of colesevelam on LDL-C, A1c and GLP-1 levels in patients with type 1 diabetes: a pilot randomized double-blind trial. Diabetes Obes Metab. 2011;13:137–43. doi:10.1111/j.1463-1326.2010.01320.x.
Marina AL, Utzschneider KM, Wright LA, et al. Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and β-cell function. Diabetes Care. 2012;35:1119–25. doi:10.2337/dc11-2050. This study show that colesevelam improves oral but not IV glucose tolerance.
Watanabe M, Morimoto K, Houten SM, et al. Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS ONE. 2012;7:e38286. doi:10.1371/journal.pone.0038286.
Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36) amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–63.
Thomson AB, Keelan M. Feeding rats diets containing cheno- or ursodeoxycholic acid or cholestyramine modifies intestinal uptake of glucose and lipids. Digestion. 1987;38:160–70.
Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–65. doi:10.1016/j.cgh.2009.10.020.
Psichas A, Little T, Lal S, McLaughlin J. Cholestyramine slows gastric emptying of liquids and reduces appetite in healthy subjects. Neurogastroenterol Motil. 2012;24:1095–101. doi:10.1111/j.1365-2982.2012.01988.x.
Maruyama T, Tanaka K, Suzuki J, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197–205. doi:10.1677/joe.1.06546.
Vassileva G, Hu W, Hoos L, et al. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J Endocrinol. 2010;205:225–32. doi:10.1677/JOE-10-0009.
Koop I, Fellgiebel A, Koop H, et al. Effect of cholestyramine on plasma cholecystokinin and pancreatic polypeptide levels, and exocrine pancreatic secretion. Eur J Clin Invest. 1988;18:517–23.
Koop I, Dorn S, Koop H, et al. Dissociation of cholecystokinin and pancreaticobiliary response to intraduodenal bile acids and cholestyramine in humans. Dig Dis Sci. 1991;36:1625–32.
Kogire M, Gomez G, Uchida T, et al. Chronic effect of oral cholestyramine, a bile salt sequestrant, and exogenous cholecystokinin on insulin release in rats. Pancreas. 1992;7:15–20.
Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–7. doi:10.2337/db08-0307.
Hofmann AF. Bile acid sequestrants improve glycemic control in type 2 diabetes: a proposed mechanism implicating glucagon-like peptide 1 release. Hepatology. 2011;53:1784. doi:10.1002/hep.24100.
Gaede P, Vedel P, Larsen N, et al. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–93. doi:10.1056/NEJMoa021778.
Gall MA, Borch-Johnsen K, Hougaard P, et al. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes. 1995;44:1303–9.
Agewall S, Wikstrand J, Ljungman S, Fagerberg B. Usefulness of microalbuminuria in predicting cardiovascular mortality in treated hypertensive men with and without diabetes mellitus. Risk Factor Intervention Study Group. Am J Cardiol. 1997;80:164–9.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Ray KK, Seshasai SRK, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomized controlled trials. Lancet. 2009;373:1765–72. doi:10.1016/S0140-6736(09)60697-8.
Compliance with Ethics Guidelines
Conflict of Interest
M. Hansen has received an unrestricted educational stipend from the Novo Nordisk Foundation. D. P. Sonne has received an unrestricted educational stipend from the Novo Nordisk Foundation. F. K. Knop has received research funding from Sanofi-Aventis Deutschland GmbH and lecture fees from AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly and Company, Gilead Sciences, Merck Sharp & Dohme, Novo Nordisk, Ono Pharmaceuticals, Sanofi, and Zealand Pharma. He is part of the Advisory Boards of Eli Lilly Denmark, Bristol-Myers Squibb/AstraZeneca, and Zealand Pharma. He has consulted for AstraZeneca, Gilead Sciences, Novo Nordisk, Ono Pharmaceuticals, and Zealand Pharma. He also has 2 pending patents.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pharmacologic Treatment of Type 2 Diabetes
Rights and permissions
About this article
Cite this article
Hansen, M., Sonne, D.P. & Knop, F.K. Bile Acid Sequestrants: Glucose-Lowering Mechanisms and Efficacy in Type 2 Diabetes. Curr Diab Rep 14, 482 (2014). https://doi.org/10.1007/s11892-014-0482-4
Published:
DOI: https://doi.org/10.1007/s11892-014-0482-4