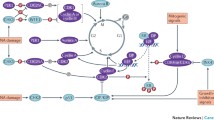
Abstract
The five individual members of the mammalian polo-like kinase family play non-redundant roles in centriole replication and maturation, mitotic progression, cytokinesis, and the DNA damage response. In human colorectal cancer, Plk1 and Plk4 are expressed at higher levels in tumor than adjacent normal mucosa, and the degree of overexpression correlates with adverse prognosis. In colorectal cancer cell lines, suppression of Plk1 activity leads to mitotic arrest and apoptosis, while inhibition of Plk4 activity reduces tumor growth and invasion. Inhibition of Plk1 or Plk4 in mice using orally bioavailable agents reduces the growth of human colorectal cancer xenografts. On the basis of these preclinical studies, clinical trials of several different targeted anti-Plk1 agents have been undertaken in patients with advanced solid tumors, and a phase I trial of the anti-Plk4 inhibitor CFI-400945 is currently accruing. Here, we review the rationale, results, and potential limitations of Plks as therapeutic targets. Evidence from genetic mouse models suggests that Plk1, Plk2, Plk3, and Plk4 may all possess tumor suppressive activity, particularly with aging. Thus, enthusiasm for the use of targeted Plk inhibitors in cancer therapy must be tempered somewhat by the potential for development of second primary tumors in the long term.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75.
Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–7.
Zitouni S, Nabais C, Jana SC, et al. Polo-like kinases: structural variations lead to multiple functions. Nat Rev Mol Cell Biol. 2014;15:433–52. A good review of recent progress in understanding the structure/function of the Plk family members.
Nam HJ, Naylor RM, van Deursen JM. Centrosome dynamics as a source of chromosomal instability. Trends Cell Biol. 2015;25:65–73.
Golsteyn RM, Schultz SJ, Bartek J, et al. Cell cycle analysis and chromosomal localization of human Plk1, a putative homologue of the mitotic kinases Drosophila polo and Saccharomyces cerevisiae Cdc5. J Cell Sci. 1994;107(Pt 6):1509–17.
Wu ZQ, Liu X. Role for Plk1 phosphorylation of Hbo1 in regulation of replication licensing. Proc Natl Acad Sci U S A. 2008;105:1919–24.
Song B, Liu XS, Davis K, Liu X. Plk1 phosphorylation of Orc2 promotes DNA replication under conditions of stress. Mol Cell Biol. 2011;31:4844–56.
Toyoshima-Morimoto F, Taniguchi E, Shinya N, et al. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature. 2001;410:215–20.
Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–13.
Joukov V, Walter JC, De Nicolo A. The Cep192-organized aurora A-Plk1 cascade is essential for centrosome cycle and bipolar spindle assembly. Mol Cell. 2014;55:578–91.
Kong D, Farmer V, Shukla A, et al. Centriole maturation requires regulated Plk1 activity during two consecutive cell cycles. J Cell Biol. 2014;206:855–65.
Casenghi M, Meraldi P, Weinhart U, et al. Polo-like kinase 1 regulates Nlp, a centrosome protein involved in microtubule nucleation. Dev Cell. 2003;5:113–25.
Feng Y, Yuan JH, Maloid SC, et al. Polo-like kinase 1-mediated phosphorylation of the GTP-binding protein Ran is important for bipolar spindle formation. Biochem Biophys Res Commun. 2006;349:144–52.
Sumara I, Gimenez-Abian JF, Gerlich D, et al. Roles of polo-like kinase 1 in the assembly of functional mitotic spindles. Curr Biol. 2004;14:1712–22.
Ahonen LJ, Kallio MJ, Daum JR, et al. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr Biol. 2005;15:1078–89.
Nishino M, Kurasawa Y, Evans R, et al. NudC is required for Plk1 targeting to the kinetochore and chromosome congression. Curr Biol. 2006;16:1414–21.
Kang YH, Park JE, Yu LR, et al. Self-regulated Plk1 recruitment to kinetochores by the Plk1-PBIP1 interaction is critical for proper chromosome segregation. Mol Cell. 2006;24:409–22.
Park CH, Park JE, Kim TS, et al. Mammalian polo-like kinase 1 (Plk1) promotes proper chromosome segregation by phosphorylating and delocalizing the PBIP1.CENP-Q complex from kinetochores. J Biol Chem. 2015;290:8569–81.
Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol. 1999;19:8625–32.
Burkard ME, Randall CL, Larochelle S, et al. Chemical genetics reveals the requirement for polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc Natl Acad Sci U S A. 2007;104:4383–8.
Fode C, Binkert C, Dennis JW. Constitutive expression of murine Sak-a suppresses cell growth and induces multinucleation. Mol Cell Biol. 1996;16:4665–72.
Kleylein-Sohn J, Westendorf J, Le Clech M, et al. Plk4-induced centriole biogenesis in human cells. Dev Cell. 2007;13:190–202.
Eckerdt F, Yamamoto TM, Lewellyn AL, Maller JL. Identification of a polo-like kinase 4-dependent pathway for de novo centriole formation. Curr Biol. 2011;21:428–32.
Hudson JW, Kozarova A, Cheung P, et al. Late mitotic failure in mice lacking Sak, a polo-like kinase. Curr Biol. 2001;11:441–6.
Rosario CO, Ko MA, Haffani YZ, et al. Plk4 is required for cytokinesis and maintenance of chromosomal stability. Proc Natl Acad Sci U S A. 2010;107:6888–93.
Holland AJ, Fachinetti D, Da Cruz S, et al. Polo-like kinase 4 controls centriole duplication but does not directly regulate cytokinesis. Mol Biol Cell. 2012;23:1838–45.
Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The polo kinase Plk4 functions in centriole duplication. Nat Cell Biol. 2005;7:1140–6.
Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, et al. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–207.
Holland AJ, Fachinetti D, Zhu Q, et al. The autoregulated instability of polo-like kinase 4 limits centrosome duplication to once per cell cycle. Genes Dev. 2012;26:2684–9.
Hiremath R, Mundaganur P, Sonwalkar P, et al. Cross sectional imaging of post partum headache and seizures. J Clin Diagn Res. 2014;8:RC01–5.
Simmons DL, Neel BG, Stevens R, et al. Identification of an early-growth-response gene encoding a novel putative protein kinase. Mol Cell Biol. 1992;12:4164–9.
Li J, Ma W, Wang PY, et al. Polo-like kinase 2 activates an antioxidant pathway to promote the survival of cells with mitochondrial dysfunction. Free Radic Biol Med. 2014;73:270–7.
Donohue PJ, Alberts GF, Guo Y, Winkles JA. Identification by targeted differential display of an immediate early gene encoding a putative serine/threonine kinase. J Biol Chem. 1995;270:10351–7.
Xu D, Yao Y, Lu L, et al. Plk3 functions as an essential component of the hypoxia regulatory pathway by direct phosphorylation of HIF-1alpha. J Biol Chem. 2010;285:38944–50.
Andrysik Z, Bernstein WZ, Deng L, et al. The novel mouse polo-like kinase 5 responds to DNA damage and localizes in the nucleolus. Nucleic Acids Res. 2010;38:2931–43.
Burns TF, Fei P, Scata KA, et al. Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol. 2003;23:5556–71.
Valenti F, Fausti F, Biagioni F, et al. Mutant p53 oncogenic functions are sustained by Plk2 kinase through an autoregulatory feedback loop. Cell Cycle. 2011;10:4330–40.
el Bahassi M, Myer DL, McKenney RJ, et al. Priming phosphorylation of Chk2 by polo-like kinase 3 (Plk3) mediates its full activation by ATM and a downstream checkpoint in response to DNA damage. Mutat Res. 2006;596:166–76.
Xie S, Wang Q, Wu H, et al. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem. 2001;276:36194–9.
Xie S, Wu H, Wang Q, et al. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J Biol Chem. 2001;276:43305–12.
Conn CW, Hennigan RF, Dai W, et al. Incomplete cytokinesis and induction of apoptosis by overexpression of the mammalian polo-like kinase, Plk3. Cancer Res. 2000;60:6826–31.
Zimmerman WC, Erikson RL. Polo-like kinase 3 is required for entry into S phase. Proc Natl Acad Sci U S A. 2007;104:1847–52.
Warnke S, Kemmler S, Hames RS, et al. Polo-like kinase-2 is required for centriole duplication in mammalian cells. Curr Biol. 2004;14:1200–7.
Chang J, Cizmecioglu O, Hoffmann I, Rhee K. PLK2 phosphorylation is critical for CPAP function in procentriole formation during the centrosome cycle. EMBO J. 2010;29:2395–406.
Krause A, Hoffmann I. Polo-like kinase 2-dependent phosphorylation of NPM/B23 on serine 4 triggers centriole duplication. PLoS One. 2010;5, e9849.
Cizmecioglu O, Krause A, Bahtz R, et al. Plk2 regulates centriole duplication through phosphorylation-mediated degradation of Fbxw7 (human Cdc4). J Cell Sci. 2012;125:981–92.
Jiang N, Wang X, Jhanwar-Uniyal M, et al. Polo box domain of Plk3 functions as a centrosome localization signal, overexpression of which causes mitotic arrest, cytokinesis defects, and apoptosis. J Biol Chem. 2006;281:10577–82.
Wang Q, Xie S, Chen J, et al. Cell cycle arrest and apoptosis induced by human polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol Cell Biol. 2002;22:3450–9.
Wang J, Beauchemin M, Bertrand R. Bcl-xL phosphorylation at Ser49 by polo kinase 3 during cell cycle progression and checkpoints. Cell Signal. 2011;23:2030–8.
de Carcer G, Escobar B, Higuero AM, et al. Plk5, a polo box domain-only protein with specific roles in neuron differentiation and glioblastoma suppression. Mol Cell Biol. 2011;31:1225–39.
Wolf G, Elez R, Doermer A, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene. 1997;14:543–9.
Dietzmann K, Kirches E. von B et al. Increased human polo-like kinase-1 expression in gliomas. J Neurooncol. 2001;53:1–11.
Strebhardt K, Kneisel L, Linhart C, et al. Prognostic value of pololike kinase expression in melanomas. JAMA. 2000;283:479–80.
Kneisel L, Strebhardt K, Bernd A, et al. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol. 2002;29:354–8.
Mito K, Kashima K, Kikuchi H, et al. Expression of polo-like kinase (PLK1) in non-Hodgkin's lymphomas. Leuk Lymphoma. 2005;46:225–31.
Yamada S, Ohira M, Horie H, et al. Expression profiling and differential screening between hepatoblastomas and the corresponding normal livers: identification of high expression of the PLK1 oncogene as a poor-prognostic indicator of hepatoblastomas. Oncogene. 2004;23:5901–11.
Macmillan JC, Hudson JW, Bull S, et al. Comparative expression of the mitotic regulators SAK and PLK in colorectal cancer. Ann Surg Oncol. 2001;8:729–40. This study showed that both Plk1 and Plk4 are more highly expressed in colorectal cancer tissue than in paired normal colonic mucosa. Plk4 declined in normal colonic mucosa with aging.
Weichert W, Kristiansen G, Schmidt M, et al. Polo-like kinase 1 expression is a prognostic factor in human colon cancer. World J Gastroenterol. 2005;11:5644–50.
Takahashi T, Sano B, Nagata T, et al. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci. 2003;94:148–52.
Knecht R, Elez R, Oechler M, et al. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:2794–7.
Knecht R, Oberhauser C, Strebhardt K. PLK (polo-like kinase), a new prognostic marker for oropharyngeal carcinomas. Int J Cancer. 2000;89:535–6.
Tokumitsu Y, Mori M, Tanaka S, et al. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol. 1999;15:687–92.
Ahr A, Karn T, Solbach C, et al. Identification of high risk breast-cancer patients by gene expression profiling. Lancet. 2002;359:131–2.
Weichert W, Kristiansen G, Winzer KJ, et al. Polo-like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Arch. 2005;446:442–50.
Wolf G, Hildenbrand R, Schwar C, et al. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract. 2000;196:753–9.
Takai N, Miyazaki T, Fujisawa K, et al. Expression of polo-like kinase in ovarian cancer is associated with histological grade and clinical stage. Cancer Lett. 2001;164:41–9.
Takai N, Miyazaki T, Fujisawa K, et al. Polo-like kinase (PLK) expression in endometrial carcinoma. Cancer Lett. 2001;169:41–9.
Weichert W, Schmidt M, Jacob J, et al. Overexpression of polo-like kinase 1 is a common and early event in pancreatic cancer. Pancreatology. 2005;5:259–65.
Gray Jr PJ, Bearss DJ, Han H, et al. Identification of human polo-like kinase 1 as a potential therapeutic target in pancreatic cancer. Mol Cancer Ther. 2004;3:641–6.
Weichert W, Schmidt M, Gekeler V, et al. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate. 2004;60:240–5.
Guan R, Tapang P, Leverson JD, et al. Small interfering RNA-mediated polo-like kinase 1 depletion preferentially reduces the survival of p53-defective, oncogenic transformed cells and inhibits tumor growth in animals. Cancer Res. 2005;65:2698–704.
Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci U S A. 2003;100:5789–94.
Gumireddy K, Reddy MV, Cosenza SC, et al. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005;7:275–86.
Spankuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K. Effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst. 2002;94:1863–77.
Reagan-Shaw S, Ahmad N. Silencing of polo-like kinase (Plk) 1 via siRNA causes induction of apoptosis and impairment of mitosis machinery in human prostate cancer cells: implications for the treatment of prostate cancer. FASEB J. 2005;19:611–3.
Spankuch-Schmitt B, Wolf G, Solbach C, et al. Downregulation of human polo-like kinase activity by antisense oligonucleotides induces growth inhibition in cancer cells. Oncogene. 2002;21:3162–71.
Steegmaier M, Hoffmann M, Baum A, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316–22.
Cogswell JP, Brown CE, Bisi JE, Neill SD. Dominant-negative polo-like kinase 1 induces mitotic catastrophe independent of cdc25C function. Cell Growth Differ. 2000;11:615–23.
Liu X, Lei M, Erikson RL. Normal cells, but not cancer cells, survive severe Plk1 depletion. Mol Cell Biol. 2006;26:2093–108.
Smith MR, Wilson ML, Hamanaka R, et al. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun. 1997;234:397–405.
Lu LY, Wood JL, Minter-Dykhouse K, et al. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol. 2008;28:6870–6.
Baran V, Solc P, Kovarikova V, et al. Polo-like kinase 1 is essential for the first mitotic division in the mouse embryo. Mol Reprod Dev. 2013;80:522–34.
Raab M, Kappel S, Kramer A, et al. Toxicity modelling of Plk1-targeted therapies in genetically engineered mice and cultured primary mammalian cells. Nat Commun. 2011;2:395.
Ma S, Charron J, Erikson RL. Role of Plk2 (Snk) in mouse development and cell proliferation. Mol Cell Biol. 2003;23:6936–43.
Villegas E, Kabotyanski EB, Shore AN, et al. Plk2 regulates mitotic spindle orientation and mammary gland development. Development. 2014;141:1562–71.
Yang Y, Bai J, Shen R, et al. Polo-like kinase 3 functions as a tumor suppressor and is a negative regulator of hypoxia-inducible factor-1 alpha under hypoxic conditions. Cancer Res. 2008;68:4077–85.
Myer DL, Robbins SB, Yin M, et al. Absence of polo-like kinase 3 in mice stabilizes Cdc25A after DNA damage but is not sufficient to produce tumors. Mutat Res. 2011;714:1–10.
Coelho PA, Bury L, Sharif B, et al. Spindle formation in the mouse embryo requires Plk4 in the absence of centrioles. Dev Cell. 2013;27:586–97.
Ko MA, Rosario CO, Hudson JW, et al. Plk4 haploinsufficiency causes mitotic infidelity and carcinogenesis. Nat Genet. 2005;37:883–8.
Harris RM, Weiss J, Jameson JL. Male hypogonadism and germ cell loss caused by a mutation in Polo-like kinase 4. Endocrinology. 2011;152:3975–85.
Sillibourne JE, Tack F, Vloemans N, et al. Autophosphorylation of polo-like kinase 4 and its role in centriole duplication. Mol Biol Cell. 2010;21:547–61.
Lingle WL, Lukasiewicz K, Salisbury JL. Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer. Adv Exp Med Biol. 2005;570:393–421.
Fujiwara T, Bandi M, Nitta M, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–7.
Basto R, Brunk K, Vinadogrova T, et al. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–42.
Liu L, Zhang CZ, Cai M, et al. Downregulation of polo-like kinase 4 in hepatocellular carcinoma associates with poor prognosis. PLoS One. 2012;7, e41293.
Rosario CO, Kazazian K, Zih FS, et al. A novel role for Plk4 in regulating cell spreading and motility. Oncogene. 2014. This is the first demonstration of the promotion of cellular migration and invasion by Plk4, with identification of Plk4 in the protrusions of motile cells.
Mason JM, Lin DC, Wei X, et al. Functional characterization of CFI-400945, a polo-like kinase 4 inhibitor, as a potential anticancer agent. Cancer Cell. 2014;26:163–76. Extensive in vitro and preclinical evidence for Plk4 as a therapeutic target in cancer. The Plk4 inhibitor CFI-400945 inhibits growth of breast cancer and colorectal cancer xenografts in mice. CFI-400945 is currently being assessed in a Phase I clinical trial.
Finetti P, Cervera N, Charafe-Jauffret E, et al. Sixteen-kinase gene expression identifies luminal breast cancers with poor prognosis. Cancer Res. 2008;68:767–76.
Agarwal R, Gonzalez-Angulo AM, Myhre S, et al. Integrative analysis of cyclin protein levels identifies cyclin b1 as a classifier and predictor of outcomes in breast cancer. Clin Cancer Res. 2009;15:3654–62.
Glinsky GV. Genomic models of metastatic cancer: functional analysis of death-from-cancer signature genes reveals aneuploid, anoikis-resistant, metastasis-enabling phenotype with altered cell cycle control and activated Polycomb Group (PcG) protein chromatin silencing pathway. Cell Cycle. 2006;5:1208–16.
Marthiens V, Rujano MA, Pennetier C, et al. Centrosome amplification causes microcephaly. Nat Cell Biol. 2013;15:731–40.
Benetatos L, Dasoula A, Hatzimichael E, et al. Polo-like kinase 2 (SNK/PLK2) is a novel epigenetically regulated gene in acute myeloid leukemia and myelodysplastic syndromes: genetic and epigenetic interactions. Ann Hematol. 2011;90:1037–45.
Li Z, Lu J, Sun M, et al. Distinct microRNA expression profiles in acute myeloid leukemia with common translocations. Proc Natl Acad Sci U S A. 2008;105:15535–40.
Smith P, Syed N, Crook T. Epigenetic inactivation implies a tumor suppressor function in hematologic malignancies for Polo-like kinase 2 but not polo-like kinase 3. Cell Cycle. 2006;5:1262–4.
Syed N, Smith P, Sullivan A, et al. Transcriptional silencing of polo-like kinase 2 (SNK/PLK2) is a frequent event in B-cell malignancies. Blood. 2006;107:250–6.
Hatzimichael E, Dasoula A, Benetatos L, et al. Study of specific genetic and epigenetic variables in multiple myeloma. Leuk Lymphoma. 2010;51:2270–4.
Pellegrino R, Calvisi DF, Ladu S, et al. Oncogenic and tumor suppressive roles of polo-like kinases in human hepatocellular carcinoma. Hepatology. 2010;51:857–68. A comprehensive study of the expression of Plks in human hepatoma. The authors suggest that Plk1 acts as an oncogene, while Plk2-4 have tumor suppressive activity. This has implications for long term use of first generation Plk1 inhibitors, which can affect Plk3 and Plk4 activity.
Ju W, Yoo BC, Kim IJ, et al. Identification of genes with differential expression in chemoresistant epithelial ovarian cancer using high-density oligonucleotide microarrays. Oncol Res. 2009;18:47–56.
de Viron E, Knoops L, Connerotte T, et al. Impaired up-regulation of polo-like kinase 2 in B-cell chronic lymphocytic leukaemia lymphocytes resistant to fludarabine and 2-chlorodeoxyadenosine: a potential marker of defective damage response. Br J Haematol. 2009;147:641–52.
Syed N, Coley HM, Sehouli J, et al. Polo-like kinase Plk2 is an epigenetic determinant of chemosensitivity and clinical outcomes in ovarian cancer. Cancer Res. 2011;71:3317–27.
Tan LB, Chen KT, Yuan YC, et al. Identification of urine PLK2 as a marker of bladder tumors by proteomic analysis. World J Urol. 2010;28:117–22.
Dai W, Li Y, Ouyang B, et al. PRK, a cell cycle gene localized to 8p21, is downregulated in head and neck cancer. Gene Chromosom Cancer. 2000;27:332–6.
Li B, Ouyang B, Pan H, et al. Prk, a cytokine-inducible human protein serine/threonine kinase whose expression appears to be down-regulated in lung carcinomas. J Biol Chem. 1996;271:19402–8.
Ando K, Ozaki T, Yamamoto H, et al. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem. 2004;279:25549–61.
Weichert W, Denkert C, Schmidt M, et al. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer. 2004;90:815–21.
Rodel F, Keppner S, Capalbo G, et al. Polo-like kinase 1 as predictive marker and therapeutic target for radiotherapy in rectal cancer. Am J Pathol. 2010;177:918–29. The authors show that higher pre-treatment Plk1 expression in rectal cancer predicts an inferior response to neoadjuvant radiation.
Sillars-Hardebol AH, Carvalho B, de Wit M, et al. Identification of key genes for carcinogenic pathways associated with colorectal adenoma-to-carcinoma progression. Tumour Biol. 2010;31:89–96.
Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24:287–91.
Liu X, Erikson RL. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc Natl Acad Sci U S A. 2002;99:8672–6.
Dohner H, Lubbert M, Fiedler W, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426–33. This report laid the foundation for Breakthrough Therapy designation from the FDA for volasertib, highlighting its potential for treating patients with acute myeloid leukemia. Based on these results, a large multicenter phase III double-blind randomized placebo-controlled trial was initiated with low-dose cytarabine ± volasertib for patients aged 65 years old or older with previously untreated AML who are ineligible for intensive remission induction therapy.
Rudolph D, Impagnatiello MA, Blaukopf C, et al. Efficacy and mechanism of action of volasertib, a potent and selective inhibitor of polo-like kinases, in preclinical models of acute myeloid leukemia. J Pharmacol Exp Ther. 2015;352:579–89.
Rudolph D, Steegmaier M, Hoffmann M, et al. BI 6727, a polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15:3094–102.
Schoffski P, Awada A, Dumez H, et al. A phase I, dose-escalation study of the novel polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer. 2012;48:179–86. First-in-man phase I dose-escalation trial of Volasertib (subsequently the first Plk1 inhibitor to proceed to phase III testing), in patients with progressive advanced or metastatic solid tumors. Preliminary evidence of antitumor activity in patients formed the basis for further clinical testing.
Stadler WM, Vaughn DJ, Sonpavde G, et al. An open-label, single-arm, phase 2 trial of the polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer. 2014;120:976–82.
Janning M, Fiedler W. Volasertib for the treatment of acute myeloid leukemia: a review of preclinical and clinical development. Future Oncol. 2014;10:1157–65.
Gjertsen BT, Schoffski P. Discovery and development of the polo-like kinase inhibitor volasertib in cancer therapy. Leukemia. 2015;29:11–9.
Schoffski P, Blay JY, De Greve J, et al. Multicentric parallel phase II trial of the polo-like kinase 1 inhibitor BI 2536 in patients with advanced head and neck cancer, breast cancer, ovarian cancer, soft tissue sarcoma and melanoma. The first protocol of the European Organization for Research and Treatment of Cancer (EORTC) Network Of Core Institutes (NOCI). Eur J Cancer. 2010;46:2206–15.
Hofheinz RD, Al-Batran SE, Hochhaus A, et al. An open-label, phase I study of the polo-like kinase-1 inhibitor, BI 2536, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:4666–74.
Sebastian M, Reck M, Waller CF, et al. The efficacy and safety of BI 2536, a novel Plk-1 inhibitor, in patients with stage IIIB/IV non-small cell lung cancer who had relapsed after, or failed, chemotherapy: results from an open-label, randomized phase II clinical trial. J Thorac Oncol. 2010;5:1060–7.
Mross K, Frost A, Steinbild S, et al. Phase I dose escalation and pharmacokinetic study of BI 2536, a novel polo-like kinase 1 inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2008;26:5511–7.
Vose JM, Friedberg JW, Waller EK, et al. The Plk1 inhibitor BI 2536 in patients with refractory or relapsed non-Hodgkin lymphoma: a phase I, open-label, single dose-escalation study. Leuk Lymphoma. 2013;54:708–13.
Ellis PM, Chu QS, Leighl N, et al. A phase I open-label dose-escalation study of intravenous BI 2536 together with pemetrexed in previously treated patients with non-small-cell lung cancer. Clin Lung Cancer. 2013;14:19–27.
Mross K, Dittrich C, Aulitzky WE, et al. A randomised phase II trial of the polo-like kinase inhibitor BI 2536 in chemo-naive patients with unresectable exocrine adenocarcinoma of the pancreas - a study within the Central European Society Anticancer Drug Research (CESAR) collaborative network. Br J Cancer. 2012;107:280–6.
Emmitte KA, Adjabeng GM, Andrews CW, et al. Design of potent thiophene inhibitors of polo-like kinase 1 with improved solubility and reduced protein binding. Bioorg Med Chem Lett. 2009;19:1694–7.
Emmitte KA, Andrews CW, Badiang JG, et al. Discovery of thiophene inhibitors of polo-like kinase. Bioorg Med Chem Lett. 2009;19:1018–21.
Olmos D, Barker D, Sharma R, et al. Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17:3420–30.
Laquerre S, Sung CM, Gilmartin AG, et al. A potent and selective Polo-like kinase 1 (Plk1) Inhibitor (GSK461364) induces cell cycle arrest and growth inhibition of cancer cell In 98th AACR Annual Meeting. Los Angeles, CA, April 14–18, Abstract number 5389: 2007.
Sutton D, Diamond M, Faucette L, et al. GSK923295A, a potent and selective CENP-E inhibitor, has broad spectrum activity against human tumor xenografts in nude mice. In 98th AACR Annual Meeting Los Angeles, CA, April 14–18, Abstract number 1522: 2007.
Garland LL, Taylor C, Pilkington DL, et al. A phase I pharmacokinetic study of HMN-214, a novel oral stilbene derivative with polo-like kinase-1-interacting properties, in patients with advanced solid tumors. Clin Cancer Res. 2006;12:5182–9.
Takagi M, Honmura T, Watanabe S, et al. In vivo antitumor activity of a novel sulfonamide, HMN-214, against human tumor xenografts in mice and the spectrum of cytotoxicity of its active metabolite, HMN-176. Investig New Drugs. 2003;21:387–99.
Tanaka H, Ohshima N, Ikenoya M, et al. HMN-176, an active metabolite of the synthetic antitumor agent HMN-214, restores chemosensitivity to multidrug-resistant cells by targeting the transcription factor NF-Y. Cancer Res. 2003;63:6942–7.
Patnaik A, Forero L, Goetz A, et al. HMN-214, a novel oral antimicrotubular agent and inhibitor of polo-like- and cyclin-dependent kinases: clinical, pharmacokinetic (PK) and pharmacodynamic (PD) relationships observed in a phase I trial of a daily × 5 schedule every 28 days. Proc Am Soc Clin Oncol. 2003; 22 Suppl: abstr 514.
Valsasina B, Beria I, Alli C, et al. NMS-P937, an orally available, specific small-molecule polo-like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol Cancer Ther. 2012;11:1006–16.
Beria I, Bossi RT, Brasca MG, et al. NMS-P937, a 4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline derivative as potent and selective Polo-like kinase 1 inhibitor. Bioorg Med Chem Lett. 2011;21:2969–74.
Ramanathan RK, Hamburg SI, Borad MJ, et al. A phase I dose escalation study of TKM-080301, a RNAi therapeutic directed against PLK1, in patients with advanced solid tumors. In 104th AACR Annual Meeting. Washington, D.C., April 6–10, Cancer Res. 73(8 Suppl) Abstract LB:289. 2013.
Bowles DW, Diamond JR, Lam ET, et al. Phase I study of oral rigosertib (ON 01910.Na), a dual inhibitor of the PI3K and Plk1 pathways, in adult patients with advanced solid malignancies. Clin Cancer Res. 2014;20:1656–65.
Jimeno A, Chan A, Cusatis G, et al. Evaluation of the novel mitotic modulator ON 01910.Na in pancreatic cancer and preclinical development of an ex vivo predictive assay. Oncogene. 2009;28:610–8.
Jimeno A, Li J, Messersmith WA, et al. Phase I study of ON 01910.Na, a novel modulator of the polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol. 2008;26:5504–10.
Ohnuma T, Lehrer D, Ren C, et al. Phase 1 study of intravenous rigosertib (ON 01910.Na), a novel benzyl styryl sulfone structure producing G2/M arrest and apoptosis, in adult patients with advanced cancer. Am J Cancer Res. 2013;3:323–38.
Ma WW, Messersmith WA, Dy GK, et al. Phase I study of Rigosertib, an inhibitor of the phosphatidylinositol 3-kinase and polo-like kinase 1 pathways, combined with gemcitabine in patients with solid tumors and pancreatic cancer. Clin Cancer Res. 2012;18:2048–55.
Komrokji RS, Raza A, Lancet JE, et al. Phase I clinical trial of oral rigosertib in patients with myelodysplastic syndromes. Br J Haematol. 2013;162:517–24.
Johnson EF, Stewart KD, Woods KW, et al. Pharmacological and functional comparison of the polo-like kinase family: insight into inhibitor and substrate specificity. Biochemistry. 2007;46:9551–63.
Reindl W, Yuan J, Kramer A, et al. Inhibition of polo-like kinase 1 by blocking polo-box domain-dependent protein-protein interactions. Chem Biol. 2008;15:459–66.
Yuan J, Sanhaji M, Kramer A, et al. Polo-box domain inhibitor poloxin activates the spindle assembly checkpoint and inhibits tumor growth in vivo. Am J Pathol. 2011;179:2091–9.
Judge AD, Robbins M, Tavakoli I, et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119:661–73.
Spankuch B, Steinhauser I, Wartlick H, et al. Downregulation of Plk1 expression by receptor-mediated uptake of antisense oligonucleotide-loaded nanoparticles. Neoplasia. 2008;10:223–34.
Steinhauser IM, Langer K, Strebhardt KM, Spankuch B. Effect of trastuzumab-modified antisense oligonucleotide-loaded human serum albumin nanoparticles prepared by heat denaturation. Biomaterials. 2008;29:4022–8.
Steinhauser I, Langer K, Strebhardt K, Spankuch B. Uptake of plasmid-loaded nanoparticles in breast cancer cells and effect on Plk1 expression. J Drug Target. 2009;17:627–37.
Sampson PB, Liu Y, Patel NK, et al. The discovery of polo-like kinase 4 inhibitors: design and optimization of Spiro[cyclopropane-1,3′[3H]indol]-2′(1′H)-ones as orally bioavailable antitumor agents. J Med Chem. 2014.
Compliance with Ethics Guidelines
Conflict of Interest
Karineh Kazazian, Olga Brashavitskaya, Francis S.W. Zih, David Berger-Richardson, Roland S.Z. Xu, Karina Pacholczyk, Jennifer Macmillan, and Carol J. Swallow declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Molecular Biology
Rights and permissions
About this article
Cite this article
Kazazian, K., Brashavitskaya, O., Zih, F.S.W. et al. Polo-Like Kinases in Colorectal Cancer: Potential for Targeted Therapy. Curr Colorectal Cancer Rep 11, 187–199 (2015). https://doi.org/10.1007/s11888-015-0275-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-015-0275-4