Abstract
Purpose of Review
This review discusses similarities and differences between cardiac positron emission tomography (PET), absolute myocardial blood flow, and flow reserve with invasive fractional flow reserve (FFR).
Recent Findings
Fundamentally, cardiac PET measures absolute myocardial blood flow whereas FFR provides a relative flow reserve. Cardiac PET offers a non-invasive and therefore lower risk alternative, able to image the entire left ventricle regardless of coronary anatomy. While cardiac PET can provide unique information about the subendocardium, FFR pullbacks offer unparalleled spatial resolution. Both diagnostic tests provide a highly repeatable and technically successful index of coronary hemodynamics that accounts for the amount of distal myocardial mass, albeit only indirectly with FFR. The randomized evidence base for FFR and its associated cost effectiveness remains unsurpassed.
Summary
Cardiac PET and FFR have been intertwined since the very development of FFR over 25 years ago. Recent work has emphasized the ability of both techniques to guide revascularization decisions by high-quality physiology. In the past few years, cardiac PET has expanded its evidence base regarding clinical outcomes, whereas FFR has solidified its position in randomized studies as the invasive reference standard.
Similar content being viewed by others
Introduction
During the past decade, coronary revascularization has moved from anatomic-driven decisions to a physiology-based approach. Whereas the COURAGE trial from 2007 [1] selected lesions using a classic, visual 70% diameter stenosis criterion—and failed to provide any benefit over medical therapy—the FAME trial from 2009 [2] treated only lesions with a reduced fractional flow reserve (FFR) and demonstrated superior clinical outcomes. An excess of 2201 citations for the FAME study in 10 years [3] and the elevation of FFR in the guidelines [4] quantify the gradual but relentless transformation. While some countries like the United States have long had a payment criterion that demanded “objective evidence of myocardial ischemia” [5], other countries like Japan introduced specific wording to this effect only in April 2018 [6] in response to the overwhelming accumulation of evidence.
But—often overlooked during the emergence of FFR—cardiac positron emission tomography (PET) with absolute blood flow quantification has shared an intimate connection. Indeed, the second ever publication on FFR [7] validated the metric for the first time in humans via relative flow reserve imaged using cardiac PET. During the 25 subsequent years, several other studies have confirmed that FFR provides an invasive measure of relative stress flow by cardiac PET [8,9,10,11]. As such, it might be tempting to assume that cardiac PET and FFR provide completely overlapping physiologic information.
Do FFR and cardiac PET simply offer alternative windows into common physiology? Should they be viewed as interchangeable metrics for informing revascularization decisions? Or do important differences exist between the physiology quantified by these diagnostic techniques? This review addresses these basic yet practical questions in light of emerging research from the past few years coupled with our own clinical experience from using both tools to treat patients in daily practice. In the following sections, we contrast FFR and cardiac PET across a range of categories, highlighting common ground, distinctions, and tradeoffs. Our goal is not to declare a winner except for patients benefiting from thoughtful, comprehensive, and personalized physiologic evaluation to determine the correct diagnosis and rational treatment.
The Cost of Coverage
Invasiveness
Both FFR and cardiac PET expose the patient to a small amount of radiation [12] and the minor risks of vasodilator medications [13,14,15], making these aspects similar. But FFR requires invasive cardiac catheterization with all its attendant upstream steps including vascular access, anticoagulation, guide catheter placement, iodine contrast injection, and coronary instrumentation. While radial access, smaller sheath and guide sizes, judicious contrast use, and modern 0.014″ pressure wires have reduced the risk, it can never reach the low levels of cardiac PET that only requires insertion of a peripheral intravenous catheter.
Specifically, coronary injury during pressure wire insertion occurs in approximately 0.1% of cases based on a survey of the literature. This rate is somewhat uncertain since many publications do not specifically list or deny adverse, wire-related events during FFR, making ascertainment bias a possibility. A brief survey of the recent literature, reflecting modern catheterization techniques and equipment, identified 8 wire-related injuries among 6181 vessels in physiology protocols [16, 17, 18], including 1 event that eventually proved fatal [18].
Patients presenting for invasive coronary angiography for other reasons—during an acute myocardial infarction or before structural heart interventions, for example—have existing indications to enter the cardiac catheterization laboratory. In these cases, the risk and equipment cost for FFR assessment add trivially to the total procedure. Indeed, skipping immediate FFR assessment for a downstream non-invasive stress test prolonged the hospital stay and increased cost in a randomized trial of patients with unstable angina [19].
Chronic Total Occlusions
In the seminal FAME trial, the protocol stated that “because FFR cannot be measured in a totally occluded artery before an intervention is performed, a default FFR value of 0.50 was recorded in the case of totally occluded arteries in the FFR group” [2]. Yet, the physiology is a chronic total occlusion (CTO) and its downstream myocardium varies greatly. As evidence, intracoronary electrographic (ECG) changes during balloon occlusion can be absent down to a collateral pressure index of approximately 0.3 in some patients [20]. This biologic heterogeneity argues against a uniform “FFR = 0.5” default as indicative of the actual intracoronary physiology or necessity of revascularization.
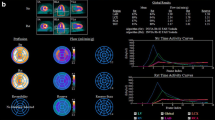
Furthermore, consider the four cases in Fig. 1, all of which represent CTOs that would have received a default value of 0.50 in FAME. PET imaging identifies that two of them would not benefit from revascularization due to a transmural infarction (matched reduction in perfusion and metabolism tracers) and minimal ischemia (normal perfusion at rest with only mild stress-induced defect). The effort and risk inherent to CTO procedures would only bring advantage for hibernating myocardium of sufficient size (perfusion reduction with intact glucose metabolism) and viable yet ischemic regions (intact resting perfusion with large, severe defect at stress).
Chronic total occlusion (CTO) anatomy versus physiology. Because invasive FFR cannot be measured in a CTO before intervention, a default value of 0.50 has been historically assumed. However, cardiac PET distinguishes among four distinct physiologic scenarios, as shown by the examples in which green arrows mark each CTO. Left: the CTO of the mid left anterior descending artery shows normal resting perfusion, indicating viable myocardium, but a large, severe defect during vasodilator stress that would be suitable for revascularization to improve angina. Center left: the CTO of the mid right coronary artery (RCA) has severely reduced resting perfusion but intact glucose metabolism, indicating hibernating myocardium appropriate for revascularization to restore ventricular function. Center right: the CTO of the mid left circumflex artery displays severe and matched reductions in resting perfusion and glucose uptake, indicating a transmural scar without benefit from revascularization. Right: the CTO of the mid RCA has normal resting perfusion, indicating viable myocardium, and only a small, mild defect during vasodilator stress due to robust collaterals from the left coronary artery that prevent ischemia
Perhaps as a result of these factors of biologic heterogeneity regarding ischemia and myocardial heterogeneity regarding tissue viability, only one of the three multicenter randomized trials of PCI for CTO showed an improvement in symptoms and all of them found no benefit for myocardial infarction or cardiac death [21,22,23]. Potentially PET-guided selection of CTOs might provide a more appropriate substrate in a future randomized trial, as suggested by PET imaging studies before versus after CTO revascularization [24, 25]. Furthermore, some CTOs are not obvious from the angiogram due to a flush occlusion and minimal collaterals, and therefore, their existence, location, and physiology can only be identified from a PET perfusion image.
Extent and Effort
Due to the intrinsic nature of the procedure, PET always images blood flow over the entire left ventricle (LV) whereas FFR studies a single vessel at a time. While FFR can be performed in multiple vessels, in practice, it remains the exception. In a large Japanese real-world registry, multivessel evaluation accounted for only 18% of the cohort [16]. Similarly, a global FFR registry noted multivessel evaluation in just under 27% of patients [26]. While the randomized trial FAME mandated multivessel disease in all subjects [2], more typical physiology trials performed multivessel FFR in only 39% [27] and 41% [28] of subjects.
A rate of 20–40% multivessel FFR evaluation from a wide literature indicates that its routine use most often interrogates just a single vessel. Because studies of mandatory multivessel FFR evaluation demonstrate significant changes in patient management [29, 30], we must assume that occult FFR positive vessels presumed negative, and unexpected FFR negative vessels assumed positive, exist with regularity. Such physiologic surprises in both directions will always be imaged with cardiac PET due to its natural and comprehensive LV coverage.
In addition to the small but non-zero risk of wire-related injury with FFR and the practical drawback to instrumenting multiple vessels, some tortuous vessels produce an “accordion” or “concertina” artifact when straightened by a wire [31]. This folding of the artery produces an iatrogenic pressure gradient from the pseudolesion that precludes valid intracoronary pressure assessment. Cardiac PET, however, images a valid and unperturbed map of myocardial flow over the entire spectrum of epicardial anatomy.
On the other hand, invasive FFR provides millimeter-level resolution regarding the location of pressure changes along the major epicardial vessels when used to create a pullback curve [32]. While a “pullback” (longitudinal gradient) by cardiac PET quantifies and predicts reduced distal invasive FFR [33], PET images have lower spatial resolution than FFR and therefore PET does not distinguish serial stenoses as sharply.
Mass and Misses
An important distinction exists between coronary flow (volume per unit time) and myocardial perfusion (flow per mass of tissue). Due to differences in supplied myocardial mass among patients for many reasons (hypertrophy from hypertension, coronary dominance, donor supply to recipient CTO, prior infarction, and male sex [34] probably due to body habitus), comparing flow provides less clarity than comparing perfusion. By nature of its flow model, cardiac PET quantifies myocardial perfusion in cc/min/g, thereby taking the distal mass into account. Similarly, FFR somewhat accounts for the amount of supplied myocardium [35] since pressure gradients depend on volumetric flow that scales with mass [36]. However, FFR does not explicitly quantify downstream mass at risk important for interventions since an FFR ≤ 0.8 in a large artery carries greater clinical weight than the same FFR for a small artery.
A tool for daily practice must perform robustly with rare failures. In the FAME trial, only 27 among 1414 lesions could not be assessed for technical reasons, implying failure in only 1.9% [2]. In two large series, cardiac PET failed in 4 of 208 (1.9%) subjects due to claustrophobia or technical issues [37], similar to 40 failures in 5373 (0.7%) consecutive cardiac PET cases from a routine clinical practice [38••]. Thus, either tool can be successfully applied in 98% or more of cases.
Peeling the Onion
Coronary stenosis does not take an equal toll on the layers of the myocardium. As well described by perfusion experiments in animals using microspheres [39, 40], the subepicardium experiences a much more mild decrease than the subendocardium in response to epicardial constriction. Indeed, the “decrease in subendocardial and increase in subepicardial flow [are] often associated with normal or even elevated total coronary blood flows so that under the circumstances of these changes, methods that measure only total left ventricular flow … give limited information” [41]. Animal experiments after a transient coronary occlusion demonstrate that subepicardial flow significantly precedes subendocardial reperfusion [42], reflecting the vulnerability of the inner myocardial layer.
Heterogeneity across the myocardium arises from the differential pressures affecting the epicardium (low and steady pericardial pressures largely reflecting intrathoracic pressure) and the endocardium (lower ventricular pressure during diastole but much higher pressure during systole). As a result, subendocardial pressure can equal or even exceed the pressure in the intramural arteries penetrating this layer, producing collapse and cessation or reversal of subendocardial flow [43]. Consequently, subendocardial perfusion becomes limited to diastole, whereas subepicardial flow continues during the entire cardiac cycle. At higher heart rates during exercise, diastole shortens at the same time that myocardial oxygen demand rises, creating the “perfect storm” for subendocardial malperfusion. The observance of angina in patients with aortic stenosis but normal coronary arteries, and its abrupt reversal after aortic valve surgery before regression of LV hypertrophy, reflects this mechanism [44].
Imaging Subendocardial Hypoperfusion
The spatial resolution necessary to distinguish subepicardial from subendocardial perfusion varies among imaging techniques. Clearly cardiac magnetic resonance (CMR) imaging offers the highest resolution currently available in humans, with an animal model reproducing the same transmyocardial perfusion gradient by imaging as simultaneously assessed by microspheres [45]. Cardiac PET has a lower resolution than CMR but can still identify transmural gradients. Work in normal subjects using oxygen-15 cardiac PET showed a subendocardial-to-subepicardial perfusion ratio of 1.12 during hyperemia [46]. Under conditions of epicardial stenosis, the same investigators [47] imaged a fall in this ratio to 0.97 when the FFR was intact (> 0.8, indicating minimal to mild epicardial disease) and fell further to 0.88 with reduced FFR (≤ 0.8, indicating moderate to severe epicardial disease).
Figure 2 displays a case example from our own clinical experience of a woman with persistent exertional dyspnea and angina but only mild coronary disease at invasive angiography. Dipyridamole hyperemia produced severe angina with 2 mm of ST segment depression without an obvious regional defect on tomographic images. However, inspection of the epicardial and endocardial borders revealed an explanatory, diffuse subendocardial perfusion defect. In her case, LV hypertrophy documented by echocardiography received reasonable flow during vasodilator stress of 2.9 cc/min/g, excluding microvascular disease, but with maldistribution to the subendocardium. Tailored, physiology-guided treatment in her case focused on control of blood pressure for regression of LV mass coupled with agents that slow the heart rate and increase diastolic perfusion time to the subendocardium.
Subendocardial angina with non-obstructive epicardial arteries. This 68-year-old woman suffered from exertional angina and dyspnea for several years, prompting an invasive angiogram that demonstrated at most mild atherosclerosis. Echocardiography demonstrated normal left ventricular (LV) function and LV hypertrophy but no significant valvular disease. Due to persistent symptoms, she underwent a cardiac PET scan with dipyridamole stress during which she developed severe angina. An excellent average stress flow of over 2.8 cc/min/g excludes microvascular dysfunction. While no regional defect is present, the inset images copy the resting epicardial and endocardial borders onto the stress tomographic images. A nearly circumferential subendocardial perfusion defect explains her symptoms and would not have been identified by epicardial assessment of FFR, flow reserve, or myocardial resistance
FFR in Subendocardial Hypoperfusion
As quantified by two, independent studies using different imaging techniques, FFR has a weak relationship with transmyocardial perfusion gradients [47, 48]. A scatterplot of FFR versus the transmyocardial perfusion gradient measured by CMR showed only a modest correlation coefficient of r = 0.63 [48]. Similarly, the scatterplot when assessing transmyocardial perfusion by cardiac PET showed a correlation coefficient of r = 0.12 [47], indicating a very weak relationship. Due to this imperfect correlation, cases will often arise with a high FFR yet subendocardial hypoperfusion. These clinical data support the statement from the animal work quoted previously that measurements of total or average transmural LV perfusion (like epicardial FFR) cannot disentangle subepicardial from subendocardial perfusion. For the case in Fig. 2, invasive epicardial evaluation with pressure or flow techniques would not reveal this subendocardial mechanism causing her symptoms. Although not measured explicitly, Fig. 2 would have had a high FFR that would correctly indicate no benefit from revascularization, but miss the mechanism for symptoms by its inability to measure maldistribution across the LV wall.
Along the range of mixed segmental and diffuse CAD from CTO to this example, FFR and PET may be concordant or discordant, both revealing true coronary pressure/flow behavior that should not be viewed as competitive methodology but rather as integrated, individualized, coronary physiology to guide thoughtful, informed management for each patient.
Flow Versus Pressure
FFR was developed as a relative flow reserve, namely hyperemic flow with stenosis relative to the maximum potential flow in the same artery without stenosis [49]. In the setting of single vessel disease, this relative flow can also be calculated by taking the ratio of hyperemic flow in the stenotic vessel to normal flow in a reference vessel [7]. Indeed, it was by this method that FFR was validated initially by PET in humans [7] after its development in an animal model [49]. Subsequently, a large literature has replicated the linear relationship between relative flow reserve by cardiac PET and invasive FFR [7,8,9,10,11].
Despite this average agreement, close inspection of FFR versus relative flow reserve scatterplots uncovers individual cases of discordance. Namely, some lesions display a large epicardial pressure gradient (and hence a low FFR) but no relative or absolute flow defect. Conversely, other lesions produce minimal pressure gradient (and hence a high FFR); yet, imaging reveals a relative or absolute flow defect.
Case Example
Consider the case in Fig. 3. This patient had a culprit lesion for his mild angina in another vessel, but let us focus on the left anterior descending (LAD) artery exclusively. Cardiac PET measured an average coronary flow reserve (CFR) over 2.7, thereby excluding microvascular disease. Serum caffeine was negative. Mean hyperemic flow exceeded 1.8 cc/min/g, far above the threshold for ischemia [50]. Relative uptake images showed neither transmural nor subendocardial perfusion defect. Regional left ventricular function remained normal. Therefore, by all objective criteria, the LAD had sufficient flow and no ischemia. However, invasive angiography for the culprit in another vessel uncovered an angiographically severe lesion with an FFR of 0.58 in the proximal LAD.
Absolute versus relative flow. This 78-year-old man had mild angina from a culprit lesion in the left circumflex, not shown since the focus is on the physiology of the left anterior descending (LAD) artery. The anterior and septal quadrants show no relative defect, either for peak uptake or transmural perfusion. Average hyperemic flow exceeded 1.8 cc/min/g, far above the thresholds for myocardial ischemia. Coronary flow reserve was over 2.7, excluding microvascular disease. Wall motion in these quadrants was normal and serum caffeine was negative. Despite intact flow to the LAD distribution, invasive angiography showed a severe, calcified lesion in the proximal vessel with an invasive FFR of 0.58. After revascularization for the angina culprit in another vessel, including a mammary artery bypass of the LAD, repeat cardiac PET showed an increase in flow to 2.8 cc/min/g, indicating a 1.8/2.8 = 0.64 relative reduction in flow similar to the pre-revascularization FFR prediction. In this case, relative flow indeed decreased by approximately 40% due to this lesion, but started from such a high level that its reduction did not produce ischemia, only a pressure gradient
To resolve this apparent discordance, recall that FFR provides an index of the relative—but not absolute—reduction in flow. In this case, after the patient underwent bypass surgery including a mammary graft to the LAD, flow in the LAD by cardiac PET increased from 1.8 to 2.8 cc/min/g, a 1.8/2.8 = 0.64 relative reduction similar to the pressure-based FFR of 0.58. Thus, flow had indeed decreased to approximately 60% of its maximum value, but started from a very high maximum such that the 40% relative reduction failed to reach ischemic thresholds, even in the subendocardium. For most patients, a 25% or greater relative reduction in flow is associated with at least 1 abnormal non-invasive test that returns to normal after revascularization [51]. But Fig. 3 reminds us that this group behavior does not hold for everyone, where the exceptions reveal true pressure/flow physiology essential for optimal, individualized management.
Pressure Gradient Versus Perfusion Defect
Further evidence for the lack of universal “ischemia” with a 20–25% relative reduction in flow comes from the CMR literature. Using stress CMR as the reference, only 17% of lesions with a so-called “gray zone” FFR between 0.75 and 0.82 had a major perfusion defect while only another 7% had a minor perfusion defect [52]. That a minority of lesions with FFR values just below the 0.80 threshold commonly used to trigger revascularization produces CMR perfusion defects perhaps explains the results of the recent MR-INFORM randomized trial [53•]. This study found equivalent clinical outcomes and freedom from angina between FFR-guided and CMR-guided revascularization, but with a lower rate of index revascularization when using CMR (35.7% versus 45.0%). Likely, the almost 10% difference in revascularization was driven by lesions with a low FFR but intact CMR perfusion whose revascularization provided no clinical advantage.
Repeatable Outcomes
Test/Retest Repeatability
Every test in clinical medicine carries imprecision due to the test itself as well as temporal, biologic variation. Immediate repeatability on the same day arises mainly from the test mechanics (equipment, assay, sensor), whereas short-term repeatability a few weeks apart also includes biologic variation.
Two large studies using modern pressure wires have shown that invasive FFR has basically no bias (average FFR difference < 0.01) and an imprecision of 0.02 (standard deviation of FFR differences) when measured a few minutes apart [54, 55]. Essentially, all studies of FFR have focused on this immediate retesting, since repeating invasive catheterization over longer periods remains prohibitive in stable patients without intervention. Hence, the short-term test/retest of FFR has not been quantified.
Several investigators have studied both the immediate test/retest of absolute flow by cardiac PET as well its short-term variation within a week or two. The largest study found no bias in paired measurements (all p values were not significant) and a same-day variation in absolute stress flow of approximately 10% using rubidium-82 that increased to about 20% variation within roughly 2 weeks [56]. Therefore, 10% variation largely represents the physics of PET imaging and flow quantification, with another 10% biologic variation over the short term.
A metric to compare repeatability among different tests must account for the distinction that absolute flow by PET exists on a ratio scale (0 cc/min/g represents a unique and non-arbitrary state) whereas FFR exists on an interval scale (since an FFR of 0.80 is not “twice as good” as an FFR of 0.40). The coefficient of variation (standard deviation of test/retest repeats divided by their mean value) commonly used to compare analytic test performance requires a ratio scale. Therefore, the ratio scale analog of FFR is the hyperemic pressure gradient (aortic pressure minus distal coronary pressure), equivalent to 1 minus FFR when dividing by the aortic pressure. In this case, a typical FFR value of 0.80 would have a normalized hyperemic pressure gradient of 0.20 so a standard deviation of 0.02 represents 10% for the coefficient of variation. Thus, the immediate test/retest repeatability of both absolute flow by cardiac PET and invasive FFR appears similar.
Clinical Outcomes and Cost Effectiveness
A well-established framework exists for evaluating diagnostic tests like cardiac PET and FFR with five criteria: technical accuracy, diagnostic accuracy, clinical pathway, patient outcomes, and cost effectiveness [57]. For the last two criteria, FFR has proven itself in a massive number of randomized controlled trials both against angiography [58, 59] and medical therapy [60•, 61]. These trials have included cost effectiveness analyses, showing it to be cost savings versus angiography [62] and cost effective versus medical therapy [63], although the tradeoff remains sensitive to the actual costs of stent and pressure wire devices that remain dynamic.
On the other hand, cardiac PET perfusion has accumulated a large amount of observational evidence regarding its association with clinical outcomes [38••, 64, 65] but limited randomized comparisons to diagnostic alternatives [66], although it has been used as an imaging endpoint in randomized therapy trials (clinicaltrials.gov NCT00756379) [67, 68]. Economic models, some from clinical trials [69] and others from observational studies [70, 71] or cost analysis [72], have largely suggested cardiac PET perfusion to be cost effective.
While randomized trials of diagnostic testing remain difficult, this challenge applies equally to FFR and cardiac PET. Given the so-called “pyramid of evidence” placing randomized trials above observational data, FFR has appropriately received a stronger level of recommendation versus imaging guidelines that largely do not distinguish among perfusion imaging alternatives [4]. Similarly, cost effectiveness analyses derived from randomized trials carry stronger weight that those derived from observational or simulation sources.
Conclusions
Health care systems have choices when creating diagnostic pathways for stable coronary artery disease. Currently, many hospitals use clinical symptoms plus traditional testing (treadmill or bicycle exercise electrocardiography, stress echocardiography, stress single-photon emission-computed tomography, or computed tomographic coronary angiography) to select patients for invasive angiography. Three drawbacks to this approach have been clearly identified. First, clinical symptoms associate weakly with the existence or absence of moderate or worse anatomic atherosclerosis, with atypical symptoms actually significantly reducing its prevalence compared to no symptoms [73]; historical pre-test probability prediction scores vastly overestimate current anatomic prevalence [74]. Second, non-invasive testing either is not performed [75], ignored even when moderately or severely abnormal such that only half of such patients are referred to invasive angiography [76], and performs poorly in practice since approximately 40% of patients with angina and a “negative” non-invasive test still have moderate to severe atherosclerosis at angiography [73]. Third, clinical judgment provides only a modest increment in uncovering physiologically obstructive disease from 6% when first ordering a non-invasive test to 27% when proceeding directly to invasive angiography based on physician discretion [77].
Given the weak or modest predictive ability of symptoms and risk scores, non-invasive testing, and clinical judgment, it is not surprising that 39% of stable patients have no or at most mild coronary disease at invasive angiography and another 23% only non-obstructive disease, leaving a minority 38% with obstructive anatomic disease [73]. This low detection rate represents an enormous opportunity to improve patient selection. Two new pathways have been undergoing development.
First, some health care systems have decided to retain their referral practice for invasive assessment but simulate FFR from the angiogram, thereby extending the tool to non-interventional catheterization laboratories plus saving wire costs and avoiding instrumentation risk. A variety of commercial tools has been developed for angiographic-derived FFR with an imprecision of ± 0.07 versus wire-based FFR [78], indicating a marked loss of diagnostic performance for lesions with a true FFR near the 0.75 to 0.80 gray zone [4]. Furthermore, the prevalence of FFR ≤ 0.80 in a pooled analysis of angiographic-simulated FFR in 1842 vessels reached only 34% [78], comparable to FFR ≤ 0.80 prevalence of 34.6% [28] and 36.8% [79] in two recent randomized outcomes trials with a total of 1257 patients. Thus these hospitals must perform approximately three invasive angiograms for one revascularization—a “conversion rate” of only one-third.
Second, other health care systems have invested in upstream “gatekeeper” imaging beyond traditional testing. Already advanced imaging tools like CMR have demonstrated themselves to be equivalent to routine invasive FFR in randomized trials [53•], and simulated FFR from computed tomographic coronary angiography (FFRCT) is undertaking an outcomes trial against standard care in the United Kingdom (clinicaltrials.gov NCT03187639). Given fewer restrictions for cardiac PET versus FFRCT and CMR, since it can be performed regardless of renal function, metallic implants, and heart rate and rhythm, more patients can pass through it as gatekeeper and avoid unnecessary invasive angiography, thereby reducing cost and increasing efficiency of the catheterization laboratory. In our own clinical experience, patients we recommend for invasive procedures based on cardiac PET have a “conversion rate” near 80%, with a minority having severe disease without a revascularization target or a CTO whose treatment remains controversial as detailed earlier in this review.
In conclusion, FFR has produced a sea-change in our approach to revascularization, moving the needle from anatomy to physiology. Cardiac PET and FFR provide fundamental tools for modern revascularization of stable coronary lesions, with similarities and differences summarized in Table 1, all reflecting true pressure/flow physiologic variability among individuals needing thoughtful, informed management specific for each patient. In our opinion, patients, physicians, hospitals, and health care systems cannot thrive without either tool.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16.
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24.
Scopus citation count for PubMed ID 19144937. Accessed September 30, 2019.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
National Coverage Determination (NCD) for Percutaneous Transluminal Angioplasty (PTA) (20.7) URL https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=201&ncdver=9&bc=BAABAAAAAAAA. Accessed September 30, 2019.
Kawase Y, Matsuo H, Akasaka T, Shiono Y, Tanaka N, Amano T, et al. Clinical use of physiological lesion assessment using pressure guidewires: an expert consensus document of the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. 2019;34(1):85–96.
De Bruyne B, Baudhuin T, Melin JA, Pijls NH, Sys SU, Bol A, et al. Coronary flow reserve calculated from pressure measurements in humans. Validation with positron emission tomography. Circulation. 1994;89(3):1013–22.
Marques KM, Knaapen P, Boellaard R, Lammertsma AA, Westerhof N, Visser FC. Microvascular function in viable myocardium after chronic infarction does not influence fractional flow reserve measurements. J Nucl Med. 2007;48(12):1987–92.
Stuijfzand WJ, Uusitalo V, Kero T, Danad I, Rijnierse MT, Saraste A, et al. Relative flow reserve derived from quantitative perfusion imaging may not outperform stress myocardial blood flow for identification of hemodynamically significant coronary artery disease. Circ Cardiovasc Imaging. 2015;8(1).
Lee JM, Kim CH, Koo BK, Hwang D, Park J, Zhang J, et al. Integrated Myocardial Perfusion Imaging Diagnostics Improve Detection of Functionally Significant Coronary Artery Stenosis by 13N-ammonia Positron Emission Tomography. Circ Cardiovasc Imaging. 2016;9(9).
Hwang D, Jeon KH, Lee JM, Park J, Kim CH, Tong Y, et al. Diagnostic performance of resting and hyperemic invasive physiological indices to Define myocardial ischemia: validation with 13N-ammonia positron emission tomography. JACC Cardiovasc Interv. 2017;10(8):751–60.
Carpeggiani C, Picano E, Brambilla M, Michelassi C, Knuuti J, Kauffman P, et al. Variability of radiation doses of cardiac diagnostic imaging tests: the RADIO-EVINCI study (RADIationdOse subproject of the EVINCI study). BMC Cardiovasc Disord. 2017;17(1):63.
Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: results from the Adenoscan multicenter trial registry. J Am Coll Cardiol. 1994;23(2):384–9.
Lette J, Tatum JL, Fraser S, Miller DD, Waters DD, Heller G, et al. Safety of dipyridamole testing in 73,806 patients: the multicenter Dipyridamole safety study. J Nucl Cardiol. 1995;2(1):3–17.
Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14(5):645–58.
Nakamura M, Yamagishi M, Ueno T, Hara K, Ishiwata S, Itoh T, et al. Prevalence of visual-functional mismatch regarding coronary artery stenosis in the CVIT-DEFER registry. Cardiovasc Interv Ther. 2014;29(4):300–8.
Ahmed N, Layland J, Carrick D, Petrie MC, McEntegart M, Eteiba H, et al. Safety of guidewire-based measurement of fractional flow reserve and the index of microvascular resistance using intravenous adenosine in patients with acute or recent myocardial infarction. Int J Cardiol. 2016;202:305–10.
Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376(13):1234–44.
Leesar MA, Abdul-Baki T, Akkus NI, Sharma A, Kannan T, Bolli R. Use of fractional flow reserve versus stress perfusion scintigraphy after unstable angina. Effect on duration of hospitalization, cost, procedural characteristics, and clinical outcome. J Am Coll Cardiol. 2003;41(7):1115–21.
Seiler C. Figure 2.85 in collateral circulation of the heart. London: Springer-Verlag; 2009.
Henriques JP, Hoebers LP, Råmunddal T, Laanmets P, Eriksen E, Bax M, et al. Percutaneous intervention for concurrent chronic Total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. 2016;68(15):1622–32.
Werner GS, Martin-Yuste V, Hildick-Smith D, Boudou N, Sianos G, Gelev V, et al. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J. 2018;39(26):2484–93.
Lee SW, Lee PH, Ahn JM, Park DW, Yun SC, Han S, et al. Randomized trial evaluating percutaneous coronary intervention for the treatment of chronic Total occlusion. Circulation. 2019;139(14):1674–83.
Stuijfzand WJ, Biesbroek PS, Raijmakers PG, Driessen RS, Schumacher SP, van Diemen P, et al. Effects of successful percutaneous coronary intervention of chronic total occlusions on myocardial perfusion and left ventricular function. EuroIntervention. 2017;13(3):345–54.
Schumacher SP, Driessen RS, Stuijfzand WJ, Raijmakers PG, Danad I, Dens J, et al. Recovery of myocardial perfusion after percutaneous coronary intervention of chronic total occlusions is comparable to hemodynamically significant non-occlusive lesions. Catheter Cardiovasc Interv. 2019;93(6):1059–66.
Schampaert E. A Global Registry of Fractional Flow Reserve (FFR)-Guided Management During Routine Clinical Procedures. SCAI in Las Vegas, Nevada. 2019.
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001.
Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376(19):1824–34.
Van Belle E, Rioufol G, Pouillot C, Cuisset T, Bougrini K, Teiger E, et al. Outcome impact of coronary revascularization strategy reclassification with fractional flow reserve at time of diagnostic angiography: insights from a large French multicenter fractional flow reserve registry. Circulation. 2014;129(2):173–85.
Curzen N, Rana O, Nicholas Z, Golledge P, Zaman A, Oldroyd K, et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain?: the RIPCORD study. Circ Cardiovasc Interv. 2014;7(2):248–55.
Deligonul U, Tatineni S, Johnson R, Kern MJ. Accordion right coronary artery: an unusual complication of PTCA guidewire entrapment. Catheter Cardiovasc Diagn. 1991;23(2):111–3.
Pijls NH, Kern MJ, Yock PG, De Bruyne B. Practice and potential pitfalls of coronary pressure measurement. Catheter Cardiovasc Interv. 2000;49(1):1–16.
Bom MJ, Driessen RS, Raijmakers PG, Everaars H, Lammertsma AA, van Rossum AC, et al. Diagnostic value of longitudinal flow gradient for the presence of haemodynamically significant coronary artery disease. Eur Heart J Cardiovasc Imaging. 2019;20(1):21–30.
Hiteshi AK, Li D, Gao Y, Chen A, Flores F, Mao SS, et al. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin Cardiol. 2014;37(10):605–9.
Leone AM, De Caterina AR, Basile E, Gardi A, Laezza D, Mazzari MA, et al. Influence of the amount of myocardium subtended by a stenosis on fractional flow reserve. Circ Cardiovasc Interv. 2013;6(1):29–36.
Johnson NP, Kirkeeide RL, Gould KL. Same Lesion, Different Artery, Different FFR!? JACC Cardiovasc Imaging. 2019;12(4):718–21.
Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, van Diemen PA, et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol. 2019;73(2):161–73.
•• Gould KL, Johnson NP, Roby AE, Nguyen T, Kirkeeide R, Haynie M, et al. Regional, artery-specific thresholds of quantitative myocardial perfusion by PET associated with reduced myocardial infarction and death after revascularization in stable coronary artery disease. J Nucl Med. 2019;60(3):410–7 This study demonstrated that a severely reduced coronary flow capacity by cardiac PET was associated with an improvement in all-cause death and myocardial infarction when revascularized instead of treated medically.
Gould KL. Assessment of coronary stenoses with myocardial perfusion imaging during pharmacologic coronary vasodilatation. IV. Limits of detection of stenosis with idealized experimental cross-sectional myocardial imaging. Am J Cardiol. 1978;42(5):761–8.
Alyono D, Anderson RW, Parrish DG, Dai XZ, Bache RJ. Alterations of myocardial blood flow associated with experimental canine left ventricular hypertrophy secondary to valvular aortic stenosis. Circ Res. 1986;58(1):47–57.
Buckberg GD, Fixler DE, Archie JP, Hoffman JI. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res. 1972;30(1):67–81.
Downey HF, Crystal GJ, Bashour FA. Asynchronous transmural perfusion during coronary reactive hyperaemia. Cardiovasc Res. 1983;17(4):200–6.
Figure 46–9 from Braunwald's Heart Disease, 9th edition (2011).
Gould KL, Carabello BA. Why angina in aortic stenosis with normal coronary arteriograms? Circulation. 2003;107(25):3121–3.
Lee DC, Simonetti OP, Harris KR, Holly TA, Judd RM, Wu E, et al. Magnetic resonance versus radionuclide pharmacological stress perfusion imaging for flow-limiting stenoses of varying severity. Circulation. 2004;110(1):58–65.
Vermeltfoort IA, Raijmakers PG, Lubberink M, Germans T, van Rossum AC, Lammertsma AA, et al. Feasibility of subendocardial and subepicardial myocardial perfusion measurements in healthy normals with (15)O-labeled water and positron emission tomography. J Nucl Cardiol. 2011;18(4):650–6.
Danad I, Raijmakers PG, Harms HJ, Heymans MW, van Royen N, Lubberink M, et al. Impact of anatomical and functional severity of coronary atherosclerotic plaques on the transmural perfusion gradient: a [15O]H2O PET study. Eur Heart J. 2014;35(31):2094–105.
Chiribiri A, Hautvast GL, Lockie T, Schuster A, Bigalke B, Olivotti L, et al. Assessment of coronary artery stenosis severity and location: quantitative analysis of transmural perfusion gradients by high-resolution MRI versus FFR. JACC Cardiovasc Imaging. 2013;6(5):600–9.
Pijls NH, van Son JA, Kirkeeide RL, De Bruyne B, Gould KL. Experimental basis of determining maximum coronary, myocardial, and collateral blood flow by pressure measurements for assessing functional stenosis severity before and after percutaneous transluminal coronary angioplasty. Circulation. 1993;87(4):1354–67.
Johnson NP, Gould KL. Physiological basis for angina and ST-segment change PET-verified thresholds of quantitative stress myocardial perfusion and coronary flow reserve. JACC Cardiovasc Imaging. 2011;4(9):990–8.
Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334(26):1703–8.
Hennigan B. A randomized controlled trial of PCI vs OMT in patients with stable angina and grey-zone fractional flow reserve values: The gzFFR study (NCT02425969). EuroPCR in Paris, France. 2018.
• Nagel E, Greenwood JP, McCann GP, Bettencourt N, Shah AM, Hussain ST, et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. N Engl J Med, 2019. 380(25):2418–28 In this randomized controlled trial, an initial perfusion imaging test was non-inferior to invasive FFR assessment, suggesting a key role for non-invasive gatekeepers to invasive cardiac catheterization.
Johnson NP, Johnson DT, Kirkeeide RL, Berry C, De Bruyne B, Fearon WF, et al. Repeatability of fractional flow reserve despite variations in systemic and coronary hemodynamics. JACC Cardiovasc Interv. 2015;8(8):1018–27.
Johnson NP, Jeremias A, Zimmermann FM, Adjedj J, Witt N, Hennigan B, et al. Continuum of vasodilator stress from rest to contrast medium to adenosine hyperemia for fractional flow reserve assessment. JACC Cardiovasc Interv. 2016;9(8):757–67.
Kitkungvan D, Johnson NP, Roby AE, Patel MB, Kirkeeide R, Gould KL. Routine clinical quantitative rest stress myocardial perfusion for managing coronary artery disease: clinical relevance of test-retest variability. JACC Cardiovasc Imaging. 2017;10(5):565–77.
Van den Bruel A, Cleemput I, Aertgeerts B, Ramaekers D, Buntinx F. The evaluation of diagnostic tests: evidence on technical and diagnostic accuracy, impact on patient outcome and cost-effectiveness is needed. J Clin Epidemiol. 2007;60(11):1116–22.
van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrøm T, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet. 2015;386(10006):1853–60.
Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36(45):3182–8.
• Zimmermann FM, Omerovic E, Fournier S, Kelbæk H, Johnson NP, Rothenbühler M, et al. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: meta-analysis of individual patient data. Eur Heart J. 2019;40(2):180–6 For the first time, this patient-level meta-analysis of three randomized controlled trials demonstrated a significant reduction in myocardial infarction when stable lesions underwent FFR-guided revascularization instead of medical therapy.
Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379(3):250–9.
Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, et al. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation. 2010;122(24):2545–50.
Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, et al. Clinical outcomes and cost-effectiveness of fractional flow reserve-guided percutaneous coronary intervention in patients with stable coronary artery disease: three-year follow-up of the FAME 2 trial (fractional flow reserve versus angiography for multivessel evaluation). Circulation. 2018;137(5):480–7.
Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62(18):1639–53.
Patel KK, Spertus JA, Chan PS, Sperry BW, Thompson RC, Al Badarin F, et al. Extent of myocardial ischemia on positron emission tomography and survival benefit with early revascularization. J Am Coll Cardiol. 2019;74(13):1645–54.
Patel KK, Al Badarin F, Chan PS, Spertus JA, Courter S, Kennedy KF, et al. Randomized comparison of clinical effectiveness of pharmacologic SPECT and PET MPI in symptomatic CAD patients. JACC Cardiovasc Imaging. 2019;12(9):1821–31.
Sdringola S, Gould KL, Zamarka LG, McLain R, Garner J. A 6 month randomized, double blind, placebo controlled, multi-center trial of high dose atorvastatin on myocardial perfusion abnormalities by positron emission tomography in coronary artery disease. Am Heart J. 2008;155(2):245–53.
Myers J, Fonda H, Vasanawala M, Chung K, Segall G, Chan K, et al. PCI alternative using sustained exercise (PAUSE): rationale and trial design. Contemp Clin Trials. 2019;79:37–43.
Langabeer JR 2nd, Delgado R, Lairson D, Johnson NP, Gould KL, Sdringola SM. Economic methods in the century trial--a comprehensive lifestyle modification study for managing coronary artery disease. J Cardiovasc Transl Res. 2012;5(3):333–6.
Merhige ME, Breen WJ, Shelton V, Houston T, D'Arcy BJ, Perna AF. Impact of myocardial perfusion imaging with PET and (82)Rb on downstream invasive procedure utilization, costs, and outcomes in coronary disease management. J Nucl Med. 2007;48(7):1069–76.
Siegrist PT, Husmann L, Knabenhans M, Gaemperli O, Valenta I, Hoefflinghaus T, et al. (13)N-ammonia myocardial perfusion imaging with a PET/CT scanner: impact on clinical decision making and cost-effectiveness. Eur J Nucl Med Mol Imaging. 2008;35(5):889–95.
Gould KL, Goldstein RA, Mullani NA. Economic analysis of clinical positron emission tomography of the heart with rubidium-82. J Nucl Med. 1989;30(5):707–17.
Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–95.
Foldyna B, Udelson JE, Karády J, Banerji D, Lu MT, Mayrhofer T, et al. Pretest probability for patients with suspected obstructive coronary artery disease: re-evaluating diamond-Forrester for the contemporary era and clinical implications: insights from the PROMISE trial. Eur Heart J Cardiovasc Imaging. 2019;20(5):574–81.
Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA. 2008;300(15):1765–73.
Hachamovitch R, Nutter B, Hlatky MA, Shaw LJ, Ridner ML, Dorbala S, et al. Patient management after noninvasive cardiac imaging results from SPARC (study of myocardial perfusion and coronary anatomy imaging roles in coronary artery disease). J Am Coll Cardiol. 2012;59(5):462–74.
Douglas PS, Pontone G, Hlatky MA, Patel MR, Norgaard BL, Byrne RA, et al. Clinical outcomes of fractional flow reserve by computed tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts study. Eur Heart J. 2015;36(47):3359–67.
Collet C, Miyazaki Y, Ryan N, Asano T, Tenekecioglu E, Sonck J, et al. Fractional flow reserve derived from computed tomographic angiography in patients with multivessel CAD. J Am Coll Cardiol. 2018;71(24):2756–69.
Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, et al. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376(19):1813–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
NPJ and KLG received internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis and have a patent pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology, unrelated to the current manuscript.
NPJ has an institutional licensing and consulting agreement with Boston Scientific for the smart minimum FFR algorithm and has received significant institutional research support from St. Jude Medical (CONTRAST, NCT02184117) and Philips Volcano Corporation (DEFINE-FLOW, NCT02328820) for studies using intracoronary pressure and flow sensors.
KLG is the 510(k) applicant for CFR Quant (K113754) and HeartSee (K143664 and K171303), software packages for cardiac positron emission tomography image processing, analysis, and absolute flow quantification.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johnson, N.P., Gould, K.L. How Do PET Myocardial Blood Flow Reserve and FFR Differ?. Curr Cardiol Rep 22, 20 (2020). https://doi.org/10.1007/s11886-020-1274-x
Published:
DOI: https://doi.org/10.1007/s11886-020-1274-x