Abstract
Purpose of Review
This review aims to summarize the role of the interleukin-1 (IL-1) blocking agents in cardiovascular diseases, briefly describing the pathogenetic rationale and the most relevant clinical studies.
Recent Findings
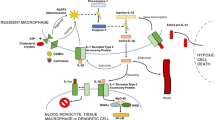
IL-1 is a pivotal cytokine of the innate immune system. Anti-IL-1 agents are currently used for the treatment of several autoimmune and autoinflammatory conditions. Recently, the role of IL-1 has also emerged in cardiovascular diseases. Indeed, two recent randomized controlled trials have shown that the IL-1 receptor antagonist anakinra is effective for the treatment of idiopathic recurrent pericarditis and the IL-1β blocking agent canakinumab is effective in reducing myocardial infarction in people at risk. Interestingly, interfering with IL-1 has proved to be also effective in other cardiovascular manifestations, such as myocarditis, arrhythmias, and heart failure.
Summary
Blocking the IL-1 pathway is a possible new therapeutic strategy, potentially leading to innovative therapies in many acute and chronic cardiovascular diseases.

Similar content being viewed by others
Abbreviations
- IL-1:
-
Interleukin-1
- NOD:
-
Nucleotide-binding domain
- NLR:
-
Nucleotide-binding domain-like receptor
- CARD:
-
Caspase activation recruitment domain
- AIM:
-
Absent in melanoma
- NLRP:
-
Nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing
- NLRC:
-
NLR-CARD (caspase activating and recruitment domain) containing
- PAMPs:
-
Pathogen-associated molecular patterns
- DAMPs:
-
Damage-associated molecular patterns
- TIR:
-
Toll/interleukin-1 receptor
- MyD88:
-
Myeloid differentiation primary response protein 88
- NF-kb:
-
Nuclear factor-kb
- MAPK:
-
Mitogen-activated protein kinase
- RA:
-
Rheumatoid arthritis
- CAPS:
-
Cryopyrin-associated periodic syndromes
- IRP:
-
Idiopathic recurrent pericarditis
- TRAPS:
-
Tumor necrosis factor receptor-1-associated periodic syndrome
- AIRTRIP:
-
Anakinra treatment of recurrent idiopathic pericarditis
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- AIR-HF:
-
Anakinra (recombinant human interleukin-1 receptor antagonist) in heart failure
- CRP:
-
C-reactive protein
- ADHF:
-
Acute decompensated heart failure
- HF:
-
Heart failure
- LVEF:
-
Left ventricle ejection fraction
- D-HART:
-
Decompensated Heart failure Anakinra Response Trial
- HFpEF:
-
HF and preserved ejection fraction
- NYHA:
-
New York Heart Association
- REDHART:
-
Recently Decompensated Heart failure Anakinra Response Trial
- MI:
-
Myocardial infarction
- CAD:
-
Coronary artery disease
- VCU-ART:
-
Virginia Commonwealth University - Anakinra Remodeling Trial
- STEMI:
-
ST segment elevation
- MR:
-
Magnetic resonance
- QTc:
-
Corrected QT
- AF:
-
Atrial fibrillation
- FMF:
-
familial Mediterranean fever
- CANTOS:
-
Canakinumab Anti-inflammatory Thrombosis Outcome Study
- IL-6:
-
Interleukin-6
References
Papers of particular interest, published recently, have been highlighted as: • Of importance•• Of major Importance
• Szekely Y, Arbel Y. A review of interleukin-1 in heart disease: where do we stand today? Cardiol Ther. 2018; https://doi.org/10.1007/s40119-018-0104-3. Relevant review focused on the role of IL-1 in heart diseases and potential therapeutic effects of anti-IL-1 treatments.
Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–18. https://doi.org/10.1016/j.immuni.2013.11.010.
• Cavalli G, Dinarello CA. Treating rheumatological diseases and co-morbidities with interleukin-1 blocking therapies. Rheumatol Oxf Engl. 2015;54(12):2134–44. https://doi.org/10.1093/rheumatology/kev269. Clinical review highlighting the role of interfering with IL-1 in cardiovascular diseases and type 2 diabetes, conditions that are frequently encountered as co-morbidities in patients with rheumatic diseases.
•• Brucato A, Imazio M, Gattorno M, Lazaros G, Maestroni S, Carraro M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA. 2016;316(18):1906–12. https://doi.org/10.1001/jama.2016.15826. First controlled, randomized trial to determine the efficacy of anakinra for colchicine-resistant and corticosteroid-dependent recurrent pericarditis.
•• Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. https://doi.org/10.1056/NEJMoa1707914. First controlled randomized double-blind trial to evaluate the efficacy of canakinumab for patients with previous myocardial infarction and a high-sensitivity C-reactive protein.
Cavalli G, Pappalardo F, Mangieri A, Dinarello CA, Dagna L, Tresoldi M. Treating life-threatening myocarditis by blocking interleukin-1. Crit Care Med. 2016;44(8):e751–4. https://doi.org/10.1097/CCM.0000000000001654.
De Jesus NM, Wang L, Lai J, Rigor RR, Francis Stuart SD, Bers DM, et al. Antiarrhythmic effects of interleukin 1 inhibition after myocardial infarction. Heart Rhythm. 2017;14(5):727–36. https://doi.org/10.1016/j.hrthm.2017.01.027.
Van Tassell BW, Raleigh JMV, Abbate A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr Heart Fail Rep. 2015;12(1):33–41. https://doi.org/10.1007/s11897-014-0231-7.
Rathinam VAK, Chan FK-M. Inflammasome, inflammation, and tissue homeostasis. Trends Mol Med. 2018;24:304–18. https://doi.org/10.1016/j.molmed.2018.01.004.
Rubartelli A. Redox control of NLRP3 inflammasome activation in health and disease. J Leukoc Biol. 2012;92(5):951–8. https://doi.org/10.1189/jlb.0512265.
Fernandes-Alnemri T, Yu J-W, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–13. https://doi.org/10.1038/nature07710.
Haneklaus M, O’Neill LAJ, Coll RC. Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: recent developments. Curr Opin Immunol. 2013;25(1):40–5. https://doi.org/10.1016/j.coi.2012.12.004.
Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–5. https://doi.org/10.1038/nri2725.
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61. https://doi.org/10.1038/nature08938.
Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, et al. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011 Feb 15;123(6):594–604. https://doi.org/10.1161/CIRCULATIONAHA.110.982777.
• Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M, et al. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. 2016;68(21):2311–28. https://doi.org/10.1016/j.jacc.2016.07.785. Relevant review focused on complicated pericarditis, in particular risk factors, pathogenetic mechanisms, management, and imaging.
Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. https://doi.org/10.1038/nri2691.
Dinarello CA, van der Meer JWM. Treating inflammation by blocking interleukin-1 in humans. Semin Immunol. 2013;25(6):469–84. https://doi.org/10.1016/j.smim.2013.10.008.
Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018 Feb;269:262–71. https://doi.org/10.1016/j.atherosclerosis.2017.12.027.
Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16(8):1000–6. https://doi.org/10.1161/01.ATV.16.8.1000.
Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, et al. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173(1):57–67. https://doi.org/10.2353/ajpath.2008.070974.
Portincasa P. Colchicine, Biologic agents and more for the treatment of familial Mediterranean fever. The old, the new, and the rare. Curr Med Chem. 2016;23(1):60–86. https://doi.org/10.2174/0929867323666151117121706.
Emmi G, Talarico R, Lopalco G, Cimaz R, Cantini F, Viapiana O, et al. Efficacy and safety profile of anti-interleukin-1 treatment in Behçet’s disease: a multicenter retrospective study. Clin Rheumatol. 2016;35(5):1281–6. https://doi.org/10.1007/s10067-015-3004-0.
Vitale A, Insalaco A, Sfriso P, Lopalco G, Emmi G, Cattalini M, et al. A snapshot on the on-label and off-label use of the interleukin-1 inhibitors in Italy among rheumatologists and pediatric rheumatologists: a nationwide multi-center retrospective observational study. Front Pharmacol. 2016;7:380. https://doi.org/10.3389/fphar.2016.00380.
Colafrancesco S, Priori R, Valesini G, Argolini L, Baldissera E, Bartoloni E, et al. Response to interleukin-1 inhibitors in 140 Italian patients with adult-onset Still’s disease: a multicentre retrospective observational study. Front Pharmacol. 2017;8:369. https://doi.org/10.3389/fphar.2017.00369.
Fabiani C, Vitale A, Emmi G, Lopalco G, Vannozzi L, Guerriero S, et al. Interleukin (IL)-1 inhibition with anakinra and canakinumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. 2017;36(1):191–7. https://doi.org/10.1007/s10067-016-3506-4.
• Lazaros G, Antonatou K, Vassilopoulos D. The therapeutic role of interleukin-1 inhibition in idiopathic recurrent pericarditis: current evidence and future challenges. Front Med. 2017;4:78. https://doi.org/10.3389/fmed.2017.00078. Clinical review by opinion leaders in the field of treatment of recurrent pericarditis.
Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921–64. https://doi.org/10.1093/eurheartj/ehv318.
Picco P, Brisca G, Traverso F, Loy A, Gattorno M, Martini A. Successful treatment of idiopathic recurrent pericarditis in children with interleukin-1beta receptor antagonist (anakinra): an unrecognized autoinflammatory disease? Arthritis Rheum. 2009;60(1):264–8. https://doi.org/10.1002/art.24174.
Massardier C, Dauphin C, Eschalier R, Lusson JR, Soubrier M. Resistant or recurrent acute pericarditis: a new therapeutic opportunity? Int J Cardiol. 2014;177(2):e75–7. https://doi.org/10.1016/j.ijcard.2014.09.192.
Cantarini L, Lucherini OM, Cimaz R, Galeazzi M. Recurrent pericarditis caused by a rare mutation in the TNFRSF1A gene and with excellent response to anakinra treatment. Clin Exp Rheumatol. 2010;28(5):802.
Emmi G, Barnini T, Silvestri E, Milco D’Elios M, Emmi L, Prisco D. A new case of idiopathic recurrent acute pericarditis due to R104Q mutation in TNFRSF1A successfully treated with anakinra: expanding the questions. Clin Exp Rheumatol. 2014;32(2):297.
Camprubí D, Mitjavila F, Arostegui JI, Corbella X. Efficacy of anakinra in an adult patient with recurrent pericarditis and cardiac tamponade as initial manifestations of tumor necrosis factor receptor-associated periodic syndrome due to the R92Q TNFRSF1A variant. Int J Rheum Dis. 2017;20(4):510–4. https://doi.org/10.1111/1756-185X.13029.
D’Elia E, Brucato A, Pedrotti P, Valenti A, De Amici M, Fiocca L, et al. Successful treatment of subacute constrictive pericarditis with interleukin-1β receptor antagonist (anakinra). Clin Exp Rheumatol. 2015;33(2):294–5.
Lazaros G, Vasileiou P, Danias P, Koutsianas C, Vlachopoulos C, Tousoulis D, et al. Effusive-constrictive pericarditis successfully treated with anakinra. Clin Exp Rheumatol. 2015 Dec;33(6):945.
Schatz A, Trankle C, Yassen A, Chipko C, Rajab M, Abouzaki N, et al. Resolution of pericardial constriction with Anakinra in a patient with effusive-constrictive pericarditis secondary to rheumatoid arthritis. Int J Cardiol. 2016;223:215–6. https://doi.org/10.1016/j.ijcard.2016.08.131.
Finetti M, Insalaco A, Cantarini L, Meini A, Breda L, Alessio M, et al. Long-term efficacy of interleukin-1 receptor antagonist (anakinra) in corticosteroid-dependent and colchicine-resistant recurrent pericarditis. J Pediatr. 2014;164(6):1425–1431.e1. https://doi.org/10.1016/j.jpeds.2014.01.065.
Jain S, Thongprayoon C, Espinosa RE, Hayes SN, Klarich KW, Cooper LT, et al. Effectiveness and safety of anakinra for management of refractory pericarditis. Am J Cardiol. 2015;116(8):1277–9. https://doi.org/10.1016/j.amjcard.2015.07.047.
Imazio M, Brucato A, Pluymaekers N, Breda L, Calabri G, Cantarini L, et al. Recurrent pericarditis in children and adolescents: a multicentre cohort study. J Cardiovasc Med (Hagerstown). 2016;17(9):707–12. https://doi.org/10.2459/JCM.0000000000000300.
Lazaros G, Vasileiou P, Koutsianas C, Antonatou K, Stefanadis C, Pectasides D, et al. Anakinra for the management of resistant idiopathic recurrent pericarditis. Initial experience in 10 adult cases. Ann Rheum Dis. 2014;73(12):2215–7. https://doi.org/10.1136/annrheumdis-2014-205990.
Lopalco G, Rigante D, Giannini M, Galeazzi M, Lapadula G, Iannone F, et al. Safety profile of anakinra in the management of rheumatologic, metabolic and autoinflammatory disorders. Clin Exp Rheumatol. 2016;34(3):531–8.
Emmi G, Silvestri E, Squatrito D, Vitale A, Bacherini D, Vannozzi L, et al. Long-term efficacy and safety of anakinra in a patient with Behçet’s disease and concomitant tuberculosis infection. Int J Dermatol. 2017;56(2):218–20.
Emmi G, Silvestri E, Cantarini L, Lopalco G, Cecchi L, Chiarini F, et al. Rapid desensitization to anakinra-related delayed reaction: need for a standardized protocol. J Dermatol. 2017;44(8):981–2. https://doi.org/10.1111/ijd.13337.
Ikonomidis I, Lekakis JP, Nikolaou M, Paraskevaidis I, Andreadou I, Kaplanoglou T, et al. Inhibition of interleukin-1 by anakinra improves vascular and left ventricular function in patients with rheumatoid arthritis. Circulation. 2008;117(20):2662–9. https://doi.org/10.1161/CIRCULATIONAHA.107.731877.
Van Tassell BW, Arena RA, Toldo S, Mezzaroma E, Azam T, Seropian IM, et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7(3):e33438. https://doi.org/10.1371/journal.pone.0033438.
• Van Tassell BW, Abouzaki NA, Oddi Erdle C, Carbone S, Trankle CR, Melchior RD, et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J Cardiovasc Pharmacol. 2016;67(6):544–51. https://doi.org/10.1097/FJC.0000000000000378. Interesting randomized, double-blinded, placebo-controlled pilot study to evaluate the role of anakinra in blocking acute inflammatory response during acute decompensated heart failure.
Van Tassell BW, Arena R, Biondi-Zoccai G, Canada JM, Oddi C, Abouzaki NA, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol. 2014;113(2):321–7. https://doi.org/10.1016/j.amjcard.2013.08.047.
Van Tassell BW, Buckley LF, Carbone S, Trankle CR, Canada JM, Dixon DL, et al. Interleukin-1 blockade in heart failure with preserved ejection fraction: rationale and design of the Diastolic Heart Failure Anakinra Response Trial 2 (D-HART2). Clin Cardiol. 2017;40(9):626–32. https://doi.org/10.1002/clc.22719.
Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail. 2017;10(11):e004373. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004373.
Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167(1):152–66. https://doi.org/10.1016/j.trsl.2015.07.002.
Ikonomidis I, Tzortzis S, Andreadou I, Paraskevaidis I, Katseli C, Katsimbri P, et al. Increased benefit of interleukin-1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7(4):619–28. https://doi.org/10.1161/CIRCIMAGING.113.001193.
Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GGL, Van Tassell BW, Robati R, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol. 2010;105(10):1371–1377.el. https://doi.org/10.1016/j.amjcard.2009.12.059.
Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111(10):1394–400. https://doi.org/10.1016/j.amjcard.2013.01.287.
• Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol. 2015;115(3):288–92. https://doi.org/10.1016/j.amjcard.2014.11.003. Valuable patient-level pooled analysis on 40 patients from two previous pilot studies, showing that anakinra may prevent new-onset heart failure after STEMI at a long-term follow-up.
• Morton AC, Rothman AMK, Greenwood JP, Gunn J, Chase A, Clarke B, et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36(6):377–84. https://doi.org/10.1093/eurheartj/ehu272. Interesting phase II, double-blinded, randomized, placebo-controlled trial showing the importance of anakinra in reducing inflammatory markers in acute coronary syndromes.
Francis Stuart SD, De Jesus NM, Lindsey ML, Ripplinger CM. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J Mol Cell Cardiol. 2016;91:114–22. https://doi.org/10.1016/j.yjmcc.2015.12.024.
Adlan AM, Panoulas VF, Smith JP, Fisher JP, Kitas GD. Association between corrected QT interval and inflammatory cytokines in rheumatoid arthritis. J Rheumatol. 2015;42(3):421–8. https://doi.org/10.3899/jrheum.140861.
Pisoni CN, Reina S, Arakaki D, Eimon A, Carrizo C, Borda E. Elevated IL-1β levels in anti-Ro/SSA connective tissue diseases patients with prolonged corrected QTc interval. Clin Exp Rheumatol. 2015;33(5):715–20.
Cheng T, Wang X-F, Hou Y-T, Zhang L. Correlation between atrial fibrillation, serum amyloid protein A and other inflammatory cytokines. Mol Med Rep. 2012;6(3):581–4. https://doi.org/10.3892/mmr.2012.934.
Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126(23):2739–48. https://doi.org/10.1161/CIRCULATIONAHA.112.122556.
Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet Lond Engl. 2018;391(10118):319–28. https://doi.org/10.1056/NEJMoa1707914.
•• Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. 2017;390(10105):1833–42. https://doi.org/10.1016/S0140-6736(17)32247-X. Intriguing exploratory analysis on the reduction of mortality by lung cancer after blockade of interleukin-1β pathway.
Neri Serneri GG, Prisco D, Martini F, Gori AM, Brunelli T, Poggesi L, et al. Acute T-cell activation is detectable in unstable angina. Circulation. 1997;95(7):1806–12.
Neri Serneri GG, Boddi M, Modesti PA, Cecioni I, Coppo M, Papa ML, et al. Immunomediated and ischemia-independent inflammation of coronary microvessels in unstable angina. Circ Res. 2003;92(12):1359–66. https://doi.org/10.1161/01.RES.0000079025.38826.E1.
Becatti M, Marcucci R, Bruschi G, Taddei N, Bani D, Gori AM, et al. Oxidative modification of fibrinogen is associated with altered function and structure in the subacute phase of myocardial infarction. Arterioscler Thromb Vasc Biol. 2014;34(7):1355–61. https://doi.org/10.1161/ATVBAHA.114.303785.
•• Becatti M, Emmi G, Silvestri E, Bruschi G, Ciucciarelli L, Squatrito D, et al. Neutrophil activation promotes fibrinogen oxidation and thrombus formation in Behçet disease. Circulation. 2016;133(3):302–11. https://doi.org/10.1161/CIRCULATIONAHA.115.017738. Important evidence that altered fibrinogen structure and impaired fibrinogen function are associated with neutrophil activation and enhanced reactive oxygen species production, suggesting a link between inflammation and thrombosis in systemic vasculitis.
Van Tassell BW, Varma A, Salloum FN, Das A, Seropian IM, Toldo S, et al. Interleukin-1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;55(2):117–22. https://doi.org/10.1097/FJC.0b013e3181c87e53.
Ridker PM, Lüscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35(27):1782–91. https://doi.org/10.1093/eurheartj/ehu203.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Giacomo Emmi has received personal fees for consultancy from GSK and for advisory board from Novartis.
Maria Letizia Urban and Silvia Maestroni declare that they have no conflict of interest.
Massimo Imazio has received an Institutional Grant for Clinical Research from SOBI.
Marco Gattorno has received fees and unrestricted grants from SOBI and Novartis.
Giuseppe Lopalco has received speaker fees from Novartis and SOBI.
Luca Cantarini has received personal fees from Novartis and SOBI.
Domenico Prisco has received grants for advisory boards from Bayer, Daichi Sankyo, Boehringer Ingelheim, BMS Pfizer, and Baxter.
Antonio Brucato has received unrestricted grants from ACARPIA and SOBI and for advisory board from SOBI.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pericardial Disease
Rights and permissions
About this article
Cite this article
Emmi, G., Urban, M.L., Imazio, M. et al. Use of Interleukin-1 Blockers in Pericardial and Cardiovascular Diseases. Curr Cardiol Rep 20, 61 (2018). https://doi.org/10.1007/s11886-018-1007-6
Published:
DOI: https://doi.org/10.1007/s11886-018-1007-6