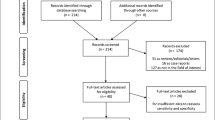
Abstract
Purpose of Review
The two most common types of cardiac amyloidosis are caused by fibril deposits of immunoglobulin light chains (AL) and transthyretin (TTR), each with distinct prognosis and clinical management. Cardiac amyloidosis is under-recognized among heart failure patients with preserved ejection fraction (HFpEF). Bone-seeking tracers like 99mTc-PYP and 99mTc-DPD have long been used to identify cardiac amyloidosis, and more recently, to differentiate TTR from AL cardiac amyloidosis in symptomatic patients. However, results are mainly derived from single-center retrospective studies, with comparable but not standardized imaging protocols and interpretation criteria.
Recent Findings
The clinical scope of cardiac amyloidosis among HFpEF patients and current literature supporting the use of bone-seeking tracers for TTR cardiac amyloidosis are presented. The differences of imaging techniques for cardiac amyloid and bone disease evaluation, bone tracer pharmacodynamics, and imaging interpretation criteria for cardiac amyloidosis diagnosis are discussed. Finally, a diagnostic algorithm to use bone scintigraphy in cardiac amyloidosis diagnosis among HFpEF patients is proposed.
Summary
Bone scintigraphy with 99mTc-PYP or 99mTc-DPD can be a useful tool with high sensitivity and specificity for detecting TTR-related cardiac amyloidosis in patients with HFpEF. It is needed to standardize the imaging protocol and interpretation criteria and to perform prospective clinical studies.
Similar content being viewed by others
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) now accounts for approximately 50% of HF patients [1, 2]. The American Heart Association and American College of Cardiology define HFpEF based on (a) signs and symptoms of HF, (b) normal left ventricular ejection fraction (LVEF), and (c) evidence of diastolic dysfunction, in the absence of other explainable causes [3]. One of these “explainable causes” is cardiac amyloidosis, often not recognized until late stages. The two most common types of cardiac amyloidosis stem from aberrant deposition of immunoglobulin light chains (AL) and the hepatically produced protein transthyretin (TTR) [4]. Deposition of un-mutated TTR causes wild-type amyloidosis, while mutant TTR aggregation results in familial amyloidosis [5]. Emerging evidence points to increasing prevalence of cardiac amyloidosis among HFpEF patients [6,7,8], yet cardiac amyloidosis might still be under-recognized as it shares many clinical characteristics with HFpEF. The gold standard for diagnosing cardiac amyloidosis is an endomyocardial biopsy, which carries a low but substantial risk of heart perforation. Therefore, clinicians need a sensitive and specific non-invasive test to properly select patients for endomyocardial biopsy or other studies in the diagnosis of cardiac amyloidosis for differentiating it from other causes of HF [9, 10].
Morphological imaging modalities such as echocardiography and cardiac magnetic resonance are commonly used to diagnose cardiac amyloidosis. However, echocardiography lacks sensitivity and specificity, though they can be improved with the addition of speckle-tracking strain measurements [11,12,13]. Speckle-tracking longitudinal strain segmental analysis shows relative apical sparing of LV wall thickening in cardiac amyloidosis which may differentiate from other causes of LV wall thickening, such as hypertensive heart disease, aortic stenosis, or rare disorders like Fabry’s disease and Friedrich’s ataxia [11]. Cardiac magnetic resonance may be useful in distinguishing cardiac amyloidosis from other causes of LV hypertrophy, but it is fraught with false-positive and false-negative findings [14]. Neither is specific enough to differentiate TTR from AL cardiac amyloidosis. It has been suggested that cerebral β-amyloid-binding positron emission tomography (PET) tracers like 18F-florbetapir, a thioflavin T-derived molecule, might be used to diagnose cardiac amyloidosis [15]. However, prospective validating studies are lacking, and this tracer cannot differentiate AL from TTR cardiac amyloidosis.
Radionuclide scintigraphy with bone-seeking tracers, such as 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), 99mTc-methylene diphosphonate (99mTc-MDP), and 99mTc-pyrophosphate (99mTc-PYP), has emerged as a valuable tool in the diagnosis of cardiac amyloidosis subtypes. Recent multicenter studies have demonstrated greater than 90% sensitivity and specificity for bone scintigraphy to distinguish TTR from AL cardiac amyloidosis [16••, 17••]. Many questions remain, however, such as appropriate-use criteria for bone scintigraphy in patients with HFpEF or asymptomatic at-risk patients. More importantly, how does bone scintigraphy aide clinicians in the diagnosis of cardiac amyloidosis among HFpEF patients? This review will (1) present the scope of the problem of finding cardiac amyloidosis in HFpEF, (2) discuss imaging protocols and possible modifications, (3) summarize the literature behind the use of bone scintigraphy for cardiac amyloidosis, and (4) propose a diagnostic algorithm to help clinicians find cardiac amyloidosis among HFpEF patients.
Prevalence of Cardiac Amyloidosis in Patients with HFpEF
Why is it important to identify cardiac amyloidosis patients early? Patients with AL cardiac amyloidosis, once symptomatic from HF, have a median survival of 8.5 months [18]. Those with TTR cardiac amyloidosis fare better, though patients with advanced wild-type TTR cardiac amyloidosis have a median survival of 43 months, while that of V122I mutant-carriers is a mere 25.6 months [19]. If identified early, individuals with HF from cardiac amyloidosis may be successfully treated. For example, in the case of AL cardiac amyloidosis, carefully selected patients undergoing orthotopic heart transplant followed by myeloablative chemotherapy and autologous stem cell transplant have the same long-term survival as those with non-amyloid HF [20, 21]. TTR amyloidosis could be treated with liver transplantation, and promising therapies ranging from TTR stabilizers to TTR silencers and degradation are already in various stages of clinical trials [5].
Why is it challenging to identify cardiac amyloidosis patients early? Cardiac amyloidosis and HFpEF have overlapping features. Both occur in older adults. The median age at diagnosis for mutant TTR and AL cardiac amyloidosis is 50 years or greater, while wild-type TTR cardiac amyloidosis tends to present in the seventh or eighth decade [22]. Age-dependent oxidation resulting in TTR tetramer destabilization and oligomerization has been proposed as one mechanism for the late presentation of wild-type TTR [5]. On the other hand, age-related vascular and ventricular stiffening is thought to contribute to the development of HFpEF [23]. It is unclear if a causal relationship exists between TTR deposition and HFpEF. Cardiac amyloidosis, however, is more common in males, while HFpEF tends to affect more women.
Left ventricular hypertrophy may be present in both cardiac amyloidosis and HFpEF, though it is rare for HFpEF patients to have very severe hypertrophy. For example, the median interventricular septal thickness in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial is 1.18 cm [24], while that of a cardiac amyloidosis cohort ranges from 1.58 to 1.88 cm [22]. Another classic feature of cardiac amyloidosis is low voltage electrocardiogram [25], which together with severe left ventricular hypertrophy, is present in late stages of the disease. Notably, up to one third of people with AL cardiac amyloidosis have no left ventricular hypertrophy at all [26], illustrating the challenge of early cardiac amyloidosis diagnosis. In a contemporary cohort of HF patients undergoing endomyocardial biopsy for standard indications, independent predictors of cardiac amyloidosis include age >50 years, BMI <30 kg/m2, presence of peripheral neuropathy, low voltage electrocardiograms, septal wall thickness ≥ 1.4 cm, and EF 50–75% [27].
Current Imaging Protocols and Interpretation Criteria
The clinical application of bone-seeking tracers for cardiac amyloidosis originates from an incidental finding of myocardial tracer accumulation on whole-body bone scans. There are three radiotracers for bone scans: 99mTc-methylene diphosphonate (99mTc-MDP), 99mTc-3,3,-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), and 99mTc-pyrophosphate (99mTc-PYP). 99mTc-MDP is currently the most commonly used tracer in the USA. 99mTc-DPD is only available in Europe but not the USA (not approved by the Food and Drug Administration). 99mTc-PYP, on the other hand, is an old bone tracer used by nuclear cardiologists for cardiac infarction imaging. Data regarding the role of bone-seeking tracers in identifying cardiac amyloidosis are mainly derived from 99mTc-PYP (USA) and 99mTc-DPD (Europe). Few studies exist on 99mTc-MDP in a small number of patients, with controversial results. It should be noted that studies on bone-seeking tracers for cardiac amyloidosis evaluation are conducted mainly based on bone scan-imaging protocols, with different imaging time, and interpretation criteria, which can affect direct comparison of results.
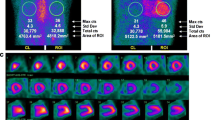
Presently, there is no consensus on how long images should be acquired after tracer injection for cardiac amyloidosis evaluation. A planar chest image is acquired either at 1 and/or 3 h after tracer injection, with or without additional single-photon emission computer tomography (SPECT) [28]. Image interpretation also varies from semi-quantitative grading score [29•], to quantitative measure of heart-to-whole body [30] or heart-to-contralateral lung uptake ratio (H/CL) [31•]. The semi-quantitative method, first proposed by Perugini et al. [29•], uses the following grading system: grade 0 = absent cardiac uptake, grade 1 = mild uptake less than bone, grade 2 = moderate uptake equal to bone, grade 3 = high uptake greater than bone (Fig. 1). Uptake ≥ grade 1 or 2 have both been considered “positive” for TTR cardiac amyloidosis in different studies, with images obtained either at 1 or 3 h after tracer injection. The quantitative H/CL ratio, on the other hand, is calculated by dividing counts from a region of interest over the heart to those of the same region on the contralateral chest wall defined as “background” (Fig. 1). H/CL ≥ 1.6 based on 1-h delayed image acquisition has been frequently used to define “positive” for TTR cardiac amyloidosis. A lower H/CL is used when images are acquired at 3 h [17••, 31•].
Representative images of 99mTc-PYP uptake in TTR cardiac amyloidosis patients. a Non visualization of cardiac uptake in an HFpEF patient without TTR involvement (arrow points to the cardiac region), grade = 0. b mild uptake less than bone, grade = 1. c Moderate uptake equal to bone, grade 2. d High uptake greater than bone, grade = 3. The circles represent region of interest in the cardiac area and contralateral right chest wall for quantitative analysis. The H/CL was calculated by dividing counts from the left to those of the same region on the contralateral chest wall (Chen W and Dilsizian V, Unpublished data)
Historically, a 3-h delayed imaging of 99mTc-PYP uptake is performed on whole-body scan for bone metastasis and bone disease evaluation, or for myocardial infarction [32, 33]. On a typical triple-phase bone scan, images obtained immediately after tracer injection (within minutes) represent blood pool or soft tissue activity, without bone visualization. Images at 3 h represent bony uptake with minimal or no soft tissue activity because of tracer elimination by renal excretion. Imaging at 1 h is not performed in a bone scan and likely represents a combination of soft tissue activity and early bone uptake.
It is unknown which time point imaging is most suitable for TTR cardiac amyloidosis visualization. For bone scans, imaging time is determined based on detailed radio-pharmacodynamical studies of the osteoblastic process accounting for high bone-to-soft tissue contrast. No such data exist for cardiac tracer uptake. The American Society of Nuclear Cardiology Practice Points document recommends 1-h delayed images for measurement of the quantitative H/CL ratio, but a 3-h delayed image acquisition for semi-quantitative grading [34]. The recommendation is based on the assumption that there is significantly increased rib uptake at 3 h compared to background which could lower H/CL ratio and potentially affect its accuracy. However, it is unknown whether the 1-h delayed image acquisition is the best time point for TTR grading.
Current Literature Supporting 99mTc-Labeled Bone-Seeking Tracers for Cardiac Amyloidosis
Accuracy
Multiple single-center retrospective studies have repeatedly shown that both 99mTc-PYP and 99mTc-DPD are very sensitive for identifying cardiac amyloidosis and able to differentiate TTR from AL with reasonable specificity. A recent study with pooled cases from three US centers showed that, among a total of 171 patients (121 TTR cardiac amyloidosis, 34 AL cardiac amyloidosis, and 16 non-amyloid HFpEF), 99mTc-PYP has a sensitivity of 88% and specificity of 88% for TTR cardiac amyloidosis, respectively, based on a semi-quantitative visual grade of 2 or above. With a heart-to-contralateral lung (H/CL) ratio > 1.6, the sensitivity is 91% and specificity 92%, respectively, for detecting TTR cardiac amyloidosis [17••]. A more recent study from an international collaboration of several renowned cardiac amyloidosis centers reported a higher accuracy for bone scintigraphy [16••]. Among a total of 1217 patients, sensitivity of a negative bone scan (including both 99mTc-PYP and 99mTc-DPD) for ruling out TTR cardiac amyloidosis is > 99%. The specificity of a positive scan is 86%. This relatively lower specificity is due mainly to mild cardiac uptake in AL patients. Specificity for TTR improves if the cutoff for positive uptake changes from grade 1 to 2. It is essential to achieve a high diagnostic specificity to avoid misdiagnosis of TTR cardiac amyloidosis in patients with AL, given the different treatments and prognoses of the two diseases. The combined finding of a positive bone scan and a negative serum or urine monoclonal protein is 100% specific for TTR cardiac amyloidosis, suggesting that bone scintigraphy may help certain patients avoid endomyocardial biopsy.
Clinical Significance
In patients with suspected cardiac amyloidosis, cardiac 99mTc-PYP uptake intensity has been shown to predict all-cause mortality and heart failure hospitalization [35]. In a subgroup of patients with confirmed diagnosis of TTR cardiac amyloidosis, neither the semi-quantitative grade nor quantitative H/CL of 99mTc-PYP uptake is associated with mortality or heart failure hospitalization. As TTR cardiac amyloidosis patients have significantly worse prognosis than those with other types of cardiomyopathy, tracer uptake intensity may not matter once TTR cardiac amyloidosis is diagnosed [35]. However, the converse has also been shown in another multicenter study, specifically that uptake intensity may correlate with worse prognosis [17••]. This is likely due to different imaging protocols, interpretation criteria, and patient populations.
Mechanism
The exact mechanism for bone-seeking tracers to visualize TTR cardiac amyloidosis is unknown. High calcium level in amyloid infiltration has been proposed to play a role, as it is associated with increased bone tracer accumulation [36]. However, many questions remain: (1) Why is tracer uptake cardiac-specific? (2) Why do bone tracers bind more strongly to TTR, not AL fibrils? (3) Why only certain tracers, such as 99mTc-PYP and 99mTc-DPD, consistently visualize TTR, but not 99mTc-MDP, even though all three tracers share the same mechanism for bone scan by incorporating into the mineral crystals? The preferential visualization of TTR is unlikely related to the amount of fibril deposition, as 99mTc-PYP uptake is significantly greater in patients with TTR than AL, despite similar wall thickness on echocardiography [31•].
Future Directions
Radio-Pharmacodynamics of Bone-Seeking Tracers in Cardiac Amyloidosis Patients
It is now commonly accepted that bone-seeking tracers are sensitive and specific tools for identifying TTR cardiac amyloidosis. Before bone scintigraphy can be applied in a broader population of HFpEF patients, it is crucial to standardize imaging protocols with respect to tracer type, radioisotope dose, and more importantly, imaging time and interpretation criteria. The current bone scan protocol may not be entirely appropriate for cardiac amyloidosis patients, some with renal insufficiency and thus decreased blood pool tracer clearance rate compared to those with bony metastases and normal renal function. Bone tracers may accumulate in bone crystals at a different rate compared to that in amyloid fibrils. More radio-pharmacodynamical studies in cardiac amyloidosis patients are needed to define the best time point that separates tracer uptake between heart and bone or soft tissues. With a standardized protocol, we can then define diagnostic criteria based on semi-quantitative scores or quantitative H/CL ratios, and finally assess their value in cardiac amyloidosis diagnosis, or predicting disease prognosis and treatment response.
Early Diagnosis of TTR Cardiac Amyloidosis in Asymptomatic Patients
Given promising therapies for TTR cardiac amyloidosis in various stages of clinical trials [5], and poor prognosis of the disease, future studies should evaluate the role of 99mTc-PYP scan in identifying and characterizing asymptomatic TTR cardiac amyloidosis, as well as its value in assessing response to new treatments and prognosis prediction. Preliminary data suggest that early diagnosis of TTR cardiac amyloidosis is possible, as accumulation of 99mTc-PYP occurs in certain patients with mild heart failure symptoms [17••]. Other studies show that bone scintigraphy may identify TTR cardiac amyloidosis even before the development of abnormalities on echocardiography or cardiac magnetic resonance [37, 38, 39••].
Mechanism of Bone-Seeking Tracers for Visualization of TTR Cardiac Amyloidosis
Examining structural differences between TTR and AL fibrils in the heart may provide clues to how various bone tracers bind TTR fibrils. Alternatively, understanding the differences in radiochemistry and radio-pharmacology between 99mTc-PYP, 99mTc-DPD, and 99mTc-MDP may explain why the former two tracers bind specifically to TTR. Knowledge of how specific tracers bind TTR versus AL fibrils may lead to the development of new tracers, perhaps one specific for AL amyloid.
Proposed Diagnostic Algorithm in Identifying Cardiac Amyloidosis
Given the increasing prevalence of cardiac amyloidosis, particularly TTR, clinicians must maintain a high index of suspicion when evaluating patients labeled as having “HFpEF.” Distinguishing AL from TTR is also of utmost importance, as the former requires chemotherapy and the latter does not. Notably, patients with TTR cardiac amyloidosis can be misdiagnosed with AL due to the presence of monoclonal gammopathy by serum protein electrophoresis with immunofixation (SPE with IFE) [40]. Serum-free κ/λ light-chain (FLC) ratios, on the other hand, are useful in identifying AL cardiac amyloidosis, with ratios < 0.2 or > 5 having 100% sensitivity and specificity in a retrospective, two-center analysis [41].
As mentioned previously, a recent multicenter, international study showed that 99mTc-PYP scintigraphy has 99% sensitivity and 86% specificity for TTR versus AL. The absence of monoclonal gammopathy by serum or urine electrophoresis, plus grade 2–3 cardiac uptake on bone scintigraphy, raises the specificity to 100% for TTR cardiac amyloidosis [16••].
Based on these findings, we propose 99mTc-PYP scintigraphy as the first diagnostic test to rule out cardiac amyloidosis in a HFpEF patient with at least one of the following characteristics: age > 50 years, peripheral neuropathy (especially bilateral carpal tunnel syndrome, orthostasis, syncope), family history of HF, low or normal voltage ECG with moderate left ventricular hypertrophy (wall thickness ≥ 1.4 cm), conduction abnormalities (first degree heart block, bundle branch block), biatrial enlargement, right ventricular hypertrophy, and pericardial effusion. The diagnostic algorithm is summarized in Fig. 2. Due to the possibility of false-positive 99mTc-PYP cardiac uptake, when there is low-grade (grade 1) uptake, endomyocardial biopsy should be strongly considered.
Diagnostic algorithm for cardiac amyloidosis identification in HFpEF. aIf grade 2–3 cardiac uptake: highly specific for TTR. bIf grade 1 cardiac uptake: EMB to confirm subtype. c,dEMB not needed if non-EMB biopsies positive for amyloid, and cMRI or TTE findings consistent with cardiac amyloidosis. dConsider TTR gene sequencing for incidental mutation. Abbreviations: EMB endomyocardial biopsy, FLC free light chains, HF heart failure, LVH left ventricular hypertrophy, SPEP with IFE serum protein electrophoresis with immunofixation
Conclusion
Bone-seeking tracers like 99mTc-PYP and 99mTc-DPD have become useful tools in the non-invasive diagnosis of cardiac amyloidosis. Knowledge gaps exist in the mechanism of tracer binding to myocardial amyloid fibrils and the lack of a uniform imaging protocol. Regardless, bone scintigraphy can be incorporated in a diagnostic algorithm to identify TTR cardiac amyloidosis among HFpEF patients. Future research should examine bone scintigraphy’s sensitivity and specificity for TTR cardiac amyloidosis in a broader HFpEF population, as well as define optimal timing of image acquisition by careful radio-pharmacodynamical experiments. Mechanistic studies on tracer binding to amyloid fibrils may not only help understand the pathophysiology of cardiac amyloidosis but also facilitate the development of better and perhaps more specific tracers for both TTR and AL. Until then, bone scintigraphy will truly be “ready for prime time” as an invaluable test in the diagnosis of cardiac amyloidosis among HFpEF patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bishu K, Redfield MM. Acute heart failure with preserved ejection fraction: unique patient characteristics and targets for therapy. Curr Heart Fail Rep. 2013;10:190–7.
Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75.
Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;2013(128):e240–327.
Gertz MA, Dispenzieri A, Sher T. Pathophysiology and treatment of cardiac amyloidosis. Nat Rev Cardiol. 2015;12:91–102.
Ton VK, Mukherjee M, Judge DP. Transthyretin cardiac amyloidosis: pathogenesis, treatments, and emerging role in heart failure with preserved ejection fraction. Clin Med Insights Cardiol. 2014;8(Suppl 1):39–44.
Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–22.
Bennani Smires Y, Victor G, Ribes D, Berry M, Cognet T, Méjean S, et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Card Imaging. 2016;32:1403–13.
Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–94.
Dilsizian V. Greatest opportunities for growth in nuclear cardiology. J Nucl Cardiol. 2017;24:1119–20.
Sengupta PP, Kramer CM, Narula J, Dilsizian V. The potential of clinical phenotyping of heart failure with imaging biomarkers for guiding therapies: A Focused Update. JACC Cardiovasc Imaging. 2017;10:1056–71.
Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–60.
Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–9.
Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–8.
Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–93.
Chen W, Dilsizian V. Molecular imaging of amyloidosis: will the heart be the next target after the brain? Curr Cardiol Rep. 2012;14:226–33.
•• Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–12. This is an international collaboration study with the largest number of cardiac amyloidosis cases from several renowned cardiac amyloidosis centers in the world. The study analyzed a total of 1217 patients with suspected cardiac amyloidosis and showed that radionuclide bone scintigraphy, was >99% sensitive and 86% specific for cardiac ATTR amyloid. The combined finding of a positive bone scan and a negative serum or urine monoclonal protein is 100% specific for TTR cardiac amyloidosis, which enables a non invasive diagnosis of cardiac TTR amyloidosis, without endomyocardial biopsy.
•• Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1:880–9. The study specifically evaluated the performance of Tc 99m PYP cardiac imaging in diagnosing cardiac TTR amyloidosis in a total of 229 suspected cardiac TTR cases from 3 academic specialty centers for cardiac amyloidosis in the United States. 99m Tc-PYP showed a sensitivity of 88% and specificity of 88% for TTR cardiac amyloidosis, respectively, based on a semi quantitative visual grade of 2 or above. With a heart-to-contralateral lung (H/CL) ratio >1.6, the sensitivity is 91% and specificity 92%, respectively, for detecting TTR cardiac amyloidosis.
Kyle RA, Gertz MA, Greipp PR, Witzig TE, Lust JA, Lacy MQ, et al. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336:1202–7.
Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the transthyretin amyloidosis cardiac study (TRACS). Am Heart J. 2012;164:222–8.
Gray Gilstrap L, Niehaus E, Malhotra R, Ton VK, Watts J, Seldin DC, et al. Predictors of survival to orthotopic heart transplant in patients with light chain amyloidosis. J Heart Lung Transplant. 2014;33:149–56.
Dey BR, Chung SS, Spitzer TR, Zheng H, MacGillivray TE, Seldin DC, et al. Cardiac transplantation followed by dose-intensive melphalan and autologous stem cell transplantation for AL amyloidosis and heart failure. Transplantation. 2010;90:905–11.
Rapezzi C, Merlini G, Quarta CC, Riva L, Longhi S, Leone O, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–12.
Upadhya B, Taffet GE, Cheng CP, Kitzman DW. Heart failure with preserved ejection fraction in the elderly: scope of the problem. J Mol Cell Cardiol. 2015;83:73–87.
Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circ Heart Fail. 2014;7:740–51.
Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114:1089–93.
Lee GY, Kim K, Choi JO, Kim SJ, Kim JS, Choe YH, et al. Cardiac amyloidosis without increased left ventricular wall thickness. Mayo Clin Proc. 2014;89:781–9.
Ton VK, Bhonsale A, Gilotra NA, Halushka MK, Steenbergen C, Almansa J, et al. Baseline characteristics predict the presence of amyloid on endomyocardial biopsy. J Card Fail. 2017;23:340–4.
Harb SC, Haq M, Flood K, Guerrieri A, Passerell W, Jaber WA, et al. National patterns in imaging utilization for diagnosis of cardiac amyloidosis: a focus on Tc99m-pyrophosphate scintigraphy. J Nucl Cardiol. 2017;24:1094–7.
• Perugini E, Guidalotti PL, Salvi F, Cooke RMT, Pettinato C, Riva L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–84. The semi-quantitative grading system was first proposed in this work to assess cardiac 99mTc-PYP uptake for the diagnosis of TTR cardiac amyloidosis: Grading system: grade 0 = absent cardiac uptake, grade 1 = mild uptake less than bone, grade 2 = moderate uptake equal to bone, grade 3 = high uptake greater than bone.
Kristen AV, Scherer K, Buss S, aus dem Siepen F, Haufe S, Bauer R, et al. Noninvasive risk stratification of patients with transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:502–10.
• Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. The quantitative heart to contralateral lung ratio (H/CL) of the region of interest was first proposed in this work to assess cardiac 99mTc-PYP uptake for the diagnosis of TTR cardiac amyloidosis: The quantitative H/CL ratio is calculated by dividing counts from a region of interest over the heart to those of the same region on the contralateral chest wall. H/CL ≥ 1.6 based on 1-hour delayed image acquisition has been frequently used to define “positive” for TTR cardiac amyloidosis.
Corbett JR, Lewis SE, Wolfe CL, Jansen DE, Lewis M, Rellas JS, et al. Measurement of myocardial infarct size by technetium pyrophosphate single-photon tomography. Am J Cardiol. 1984;54:1231–6.
Rude RE, Parkey RW, Bonte FJ, Lewis SE, Twieg D, Buja LM, et al. Clinical implications of the technetium-99m stannous pyrophosphate myocardial scintigraphic “doughnut” pattern in patients with acute myocardial infarcts. Circulation. 1979;59:721–30.
Dorbala S, Bokhari S, Miller E et al. American Society of Nuclear Cardiology. Practice points: 99m technetium-pyrophosphate imaging for transthyretin cardiac amyloidosis. http://www.ASNC.org.
Vranian MN, Sperry BW, Hanna M, et al. Technetium pyrophosphate uptake in transthyretin cardiac amyloidosis: associations with echocardiographic disease severity and outcomes. Journal of nuclear cardiology. J Nucl Cardiol. 2017. https://doi.org/10.1007/s12350-016-0768-9.
Willerson JT, Parkey RW, Bonte FJ, Lewis SE, Corbett J, Maximilian Buja L. Pathophysiologic considerations and clinicopathological correlates of technetium-99m stannous pyrophosphate myocardial scintigraphy. Semin Nucl Med. 1980;10:54–69.
Glaudemans AW, van Rheenen RW, van den Berg MP, et al. Bone scintigraphy with (99m)technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid. 2014;21:35–44.
Fontana M, Banypersad SM, Treibel TA, Maestrini V, Sado DM, White SK, et al. Native T1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157–65.
•• Haq M, Pawar S, Berk JL, Miller EJ, Ruberg FL. Can 99mTc-pyrophosphate Aid in Early Detection of Cardiac involvement in asymptomatic variant TTR amyloidosis? JACC Cardiovasc Imaging. 2017;10:713–4. This study compared the cardiac 99mTc-PYP uptake in 3 groups of patients: group 1: nonamyloid HFpEF; group 2: asymptomatic TTR mutation carriers; and group 3: TTR mutation and symptomatic heart failure. Although sample number is relatively small in each group, the study showed that abnormal cardiac uptake of 99mTc-PYP by both qualitative and semi-quantitative methods preceded overt echocardiographic, cardiac biomarker, or clinical signs among asymptomatic TTR mutation carriers. The results suggested that 99mTc-PYP scintigraphy may be the first measurable manifestation of TTR cardiac amyloid which may permit early diagnosis and intervention of the disease.
Lachmann HJ, Booth DR, Booth SE, Bybee A, Gilbertson JA, Gillmore JD, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med. 2002;346:1786–91.
Halushka MK, Eng G, Collins AB, Judge DP, Semigran MJ, Stone JR. Optimization of serum immunoglobulin free light chain analysis for subclassification of cardiac amyloidosis. J Cardiovasc Transl Res. 2015;8:264–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Wengen Chen, Van-Khue Ton, and Vasken Dilsizian declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nuclear Cardiology
Rights and permissions
About this article
Cite this article
Chen, W., Ton, VK. & Dilsizian, V. Clinical Phenotyping of Transthyretin Cardiac Amyloidosis with Bone-Seeking Radiotracers in Heart Failure with Preserved Ejection Fraction. Curr Cardiol Rep 20, 23 (2018). https://doi.org/10.1007/s11886-018-0970-2
Published:
DOI: https://doi.org/10.1007/s11886-018-0970-2