Abstract
Purpose of Review
This forward-looking review summarizes existing evidence from cardiovascular outcome trials on cardiometabolic risk-reduction in type 2 diabetes (T2DM) management, with attention to updating and personalizing recommendations from recent diabetes practice guidelines issued by cardiology societies.
Recent Findings
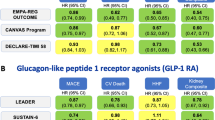
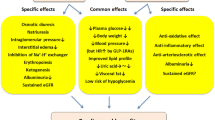
T2DM management has shifted towards cardiometabolic outcome improvement rather than purely glycemic control. According to large clinical trials, sodium-glucose cotransporter-2 inhibitors showed robust results in reducing heart failure (HF) hospitalization and chronic kidney disease (CKD) progression, while glucagon-like peptide-1 receptor agonists demonstrated the largest effects on HbA1c reduction, weight loss, and atherosclerotic cardiovascular disease outcomes prevention, including stroke.
Summary
Considering the distinct features of these new cardiometabolic agents, initial selection of therapy should be targeted to each individual patient, with consideration of combination therapy for the highest risk patients. Moreover, future studies should investigate the addition of obesity-predominant risk, in conjunction with coronary artery disease, stroke, CKD, and HF, as a new influential indicator for choosing the optimal cardiometabolic agent.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–28. https://doi.org/10.2337/dci18-0007.
Waters H GM: America’s obesity crisis: the health and economic costs of excess weight. Milken Institute. https://www.milkeninstitute.org/reports/americas-obesity-crisis-health-and-economic-costs-excess-weight (October 2018). Accessed November 22 2021.
Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care. 2004;27(7):1605–9. https://doi.org/10.2337/diacare.27.7.1605.
McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2(10):843–51. https://doi.org/10.1016/s2213-8587(14)70031-2.
Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health. 1991;81(9):1158–62. https://doi.org/10.2105/ajph.81.9.1158.
Kranenburg G, van der Graaf Y, van der Leeuw J, Nathoe HM, de Borst GJ, Kappelle LJ, et al. The relation between HbA1c and cardiovascular events in patients with type 2 diabetes with and without vascular disease. Diabetes Care. 2015;38(10):1930–6. https://doi.org/10.2337/dc15-0493.
Sarwar N, Aspelund T, Eiriksdottir G, Gobin R, Seshasai SR, Forouhi NG, et al. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 2010;7(5):e1000278. https://doi.org/10.1371/journal.pmed.1000278.
Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35(2):446–54. https://doi.org/10.2337/dc11-1465.
Apolzan JW, Venditti EM, Edelstein SL, Knowler WC, Dabelea D, Boyko EJ, et al. Long-term weight loss with metformin or lifestyle intervention in the diabetes prevention program outcomes study. Ann Intern Med. 2019;170(10):682–90. https://doi.org/10.7326/M18-1605.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65.
Lamanna C, Monami M, Marchionni N, Mannucci E. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2011;13(3):221–8. https://doi.org/10.1111/j.1463-1326.2010.01349.x.
Medicine. USNLo: Investigation of metformin in pre-diabetes on atherosclerotic cardiovascular outcomes. https://clinicaltrials.gov/show/NCT02915198 Accessed 23 November 2021.
Hasan SS, Aslam Q, Islam I, Kow CS, Babar ZUD. Metformin-based single pill drug combinations for type 2 diabetes in primary care England: a time trend analysis. Prim Care Diabetes. 2022;16(2):271–8. https://doi.org/10.1016/j.pcd.2022.01.008.
Standards of medical care in diabetes-2018 abridged for primary care providers. Clin Diabetes. 2018;36(1):14–37. https://doi.org/10.2337/cd17-0119.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. https://doi.org/10.1016/s0140-6736(19)31149-3.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. https://doi.org/10.1016/s0140-6736(18)32261-x.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827.
Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. https://doi.org/10.1056/NEJMc1712572.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720.
• Goldberg RB, Orchard TJ, Crandall JP, Boyko EJ, Budoff M, Dabelea D, et al. Effects of long-term metformin and lifestyle interventions on cardiovascular events in the diabetes prevention program and its outcome study. Circulation. 2022;145(22):1632–41. https://doi.org/10.1161/circulationaha.121.056756. This study provides evidence that metformin does not have CV benefits which propel the T2DM treatment towards SGLT2is and GLP1-RAs.
Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens. 2015;24(5):463–9. https://doi.org/10.1097/MNH.0000000000000152.
Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5(6):632–44. https://doi.org/10.1016/j.jacbts.2020.02.004.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61. https://doi.org/10.1056/NEJMoa2107038.
Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35. https://doi.org/10.1056/NEJMoa2004967.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. https://doi.org/10.1056/NEJMoa2024816.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303.
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–24. https://doi.org/10.1056/NEJMoa2022190.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. https://doi.org/10.1056/NEJMoa1811744.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. https://doi.org/10.1056/NEJMoa1812389.
Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium glucose cotransporter-2 inhibition in heart failure: potential mechanisms, clinical applications, and summary of clinical trials. Circulation. 2017;136(17):1643–58. https://doi.org/10.1161/circulationaha.117.030012.
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–93. https://doi.org/10.2337/dc15-0843.
Legaspi R, Narciso P. Euglycemic diabetic ketoacidosis due to gastroparesis, a local experience. J Ark Med Soc. 2015;112(5):62–3.
Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28. https://doi.org/10.1056/NEJMoa2030183.
Li N, Lv D, Zhu X, Wei P, Gui Y, Liu S, et al. Effects of SGLT2 inhibitors on renal outcomes in patients with chronic kidney disease: a meta-analysis. Front Med (Lausanne). 2021;8: 728089. https://doi.org/10.3389/fmed.2021.728089.
Cavender MA, Norhammar A, Birkeland KI, Jørgensen ME, Wilding JP, Khunti K, et al. SGLT-2 inhibitors and cardiovascular risk: an analysis of CVD-REAL. J Am Coll Cardiol. 2018;71(22):2497–506. https://doi.org/10.1016/j.jacc.2018.01.085.
Kosiborod M, Lam CSP, Kohsaka S, Kim DJ, Karasik A, Shaw J, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71(23):2628–39. https://doi.org/10.1016/j.jacc.2018.03.009.
Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. https://doi.org/10.1056/NEJMoa2108269.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. https://doi.org/10.1056/NEJMoa1612917.
Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–51. https://doi.org/10.1056/NEJMoa1901118.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. https://doi.org/10.1056/NEJMoa1509225.
Garber AJ. GLP-1 receptor agonists: why is once weekly inferior to once daily? Lancet Diabetes Endocrinol. 2014;2(4):266–7. https://doi.org/10.1016/s2213-8587(14)70014-2.
Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105–13. https://doi.org/10.1016/s2213-8587(17)30412-6.
Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20(1):189. https://doi.org/10.1186/s12933-021-01366-8.
FDANewsRelease.: FDA approves Jardiance to reduce cardiovascular death in adults with type 2 diabetes. . https://www.fda.gov/news-events/press-announcements/fda-approves-jardiance-reduce-cardiovascular-death-adults-type-2-diabetes (2016). Accessed November 30 2021.
FDANewsRelease.: Liraglutide Approved for MACE in T2D With Established CVD. . https://www.endocrinologyadvisor.com/home/topics/diabetes/type-2-diabetes/fda-liraglutide-approved-for-mace-in-t2d-with-established-cvd/ (2017). Accessed November 30 2021.
FDANewsRelease.: FDA approves new treatment for a type of heart failure. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure (2020). Accessed November 30 2021.
FDANewsRelease.: FDA approves dulaglutide for adults with T2D, regardless of CVD. https://www.ajmc.com/view/fda-approves-dulaglutide-for-adults-with-t2d-regardless-of-cvd (2020). Accessed November 30 2021.
Administration FaD: FDA approves treatment for wider range of patients with heart failure. https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-wider-range-patients-heart-failure (2022). Accessed.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. https://doi.org/10.1093/eurheartj/ehz486.
Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL Jr, Kalyani RR, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117–45. https://doi.org/10.1016/j.jacc.2020.05.037.
Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol. 2021. https://doi.org/10.1093/eurjpc/zwab154
•• Committee ADAPP. 10. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Supplement_1):S144-S74. https://doi.org/10.2337/dc22-S010. This recent guideline (despite the 2021 version) proposed utilizing SGLT2is and GLP1-RAs as the first agent for T2DM treatment in patients with established ASCVD, HF, CKD, and high CV risk.
Li C, Luo J, Jiang M, Wang K. The efficacy and safety of the combination therapy with GLP-1 receptor agonists and SGLT-2 inhibitors in type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol. 2022;13:838277-. https://doi.org/10.3389/fphar.2022.838277.
Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. https://doi.org/10.1056/NEJMoa2108269.
ClinicalTrials.gov.: a research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). Identifier: NCT03819153. https://clinicaltrials.gov/ct2/show/NCT03819153 Accessed.
ClinicalTrials.gov.: renal effects of treatment with empagliflozin alone or in combination with semaglutide in patients with type 2 diabetes and albuminuria (EmpaSema). Identifier: NCT04061200. https://clinicaltrials.gov/ct2/show/NCT04061200 Accessed.
PCORI.org: PRECIDENTD (PREvention of CardIovascular and DiabEtic kidNey disease in Type 2 Diabetes). https://www.pcori.org/research-results/2021/precidentd-prevention-cardiovascular-and-diabetic-kidney-disease-type-2 (2021). Accessed November 20 2021.
Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. 2015;65(8):846–55. https://doi.org/10.1016/j.jacc.2014.11.041.
Cardoso R, Dudum R, Ferraro RA, Bittencourt M, Blankstein R, Blaha MJ, et al. Cardiac computed tomography for personalized management of patients with type 2 diabetes mellitus. Circ Cardiovasc Imaging. 2020;13(9):e011365. https://doi.org/10.1161/circimaging.120.011365.
Elkeles RS, Godsland IF, Feher MD, Rubens MB, Roughton M, Nugara F, et al. Coronary calcium measurement improves prediction of cardiovascular events in asymptomatic patients with type 2 diabetes: the PREDICT study. Eur Heart J. 2008;29(18):2244–51. https://doi.org/10.1093/eurheartj/ehn279.
Malik S, Zhao Y, Budoff M, Nasir K, Blumenthal RS, Bertoni AG, et al. Coronary artery calcium score for long-term risk classification in individuals with type 2 diabetes and metabolic syndrome from the multi-ethnic study of atherosclerosis. JAMA Cardiol. 2017;2(12):1332–40. https://doi.org/10.1001/jamacardio.2017.4191.
Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014;7(2):282–91. https://doi.org/10.1161/circimaging.113.001047.
Cainzos-Achirica M, Patel KV, Quispe R, Joshi PH, Khera A, Ayers C, et al. Coronary artery calcium for the allocation of GLP-1RA for primary prevention of atherosclerotic cardiovascular disease. JACC Cardiovasc Imaging. 2021;14(7):1470–2. https://doi.org/10.1016/j.jcmg.2020.12.024.
Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2110956.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29. https://doi.org/10.1056/NEJMoa2025845.
R A. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. European Heart Journal. 2021. https://doi.org/10.1093/eurheartj/ehab777.
FDANewsRelease.: FDA approves drug to reduce risk of serious kidney and heart complications in adults with chronic kidney disease associated with type 2 diabetes. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-reduce-risk-serious-kidney-and-heart-complications-adults-chronic-kidney-disease (2021). Accessed November 30 2021.
Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811–24. https://doi.org/10.1016/s0140-6736(21)02188-7.
Adminestration USFD: FDA approves novel, dual-targeted treatment for type 2 diabetes. https://www.fda.gov/news-events/press-announcements/fda-approves-novel-dual-targeted-treatment-type-2-diabetes (2022). Accessed.
Harris ST, Patorno E, Zhuo M, Kim SC, Paik JM. Prescribing trends of antidiabetes medications in patients with type 2 diabetes and diabetic kidney disease, a cohort study. Diabetes Care. 2021. https://doi.org/10.2337/dc21-0529.
Adhikari R, Jha K, Dardari Z, Heyward J, Blumenthal RS, Eckel RH, et al. National trends in use of sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists by cardiologists and other specialties, 2015 to 2020. J Am Heart Assoc. 2022;11(9):e023811. https://doi.org/10.1161/JAHA.121.023811.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Blaha Grants: NIH, FDA, AHA, Amgen, Novo Nordisk, Bayer. Advisory Boards: Amgen, Novartis, Novo Nordisk, Bayer, Roche, 89Bio, Kaleido, Inozyme, Agepha. Consulting: Kowa, emocha
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jha, K.K., Adhikari, R., Tasdighi, E. et al. Transitioning to GLP-1 RAs and SGLT2 Inhibitors as the First Choice for Managing Cardiometabolic Risk in Type 2 Diabetes. Curr Atheroscler Rep 24, 925–937 (2022). https://doi.org/10.1007/s11883-022-01066-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11883-022-01066-y