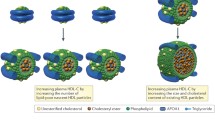
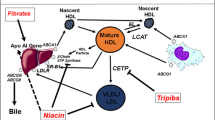
Abstract
Several recent reports have raised doubts about the atheroprotective role of high-density lipoprotein cholesterol (HDL-C). Nevertheless, a substantial body of work supports the validity of pharmacological interventions able to enhance HDL function, as opposed to raising HDL-C levels per se. In this article, we briefly review the development of pharmacological interventions that target apoA-I and HDL function as a means of reducing atherosclerotic risk: small molecule pharmaceuticals, small HDL mimetic peptides, and infusion of apoA-I-containing particles.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62(5):707–14.
Sirtori CR, Calabresi L, Franceschini G, Baldassarre D, Amato M, Johansson J, et al. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation. 2001;103(15):1949–54.
Calabresi L, Simonelli S, Gomaraschi M, Franceschini G. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 2012;222(2):299–306.
Haase CL, Tybjaerg-Hansen A, Qayyum AA, Schou J, Nordestgaard BG, Frikke-Schmidt R. LCAT, HDL cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J Clin Endocrinol Metab. 2012;97(2):E248–256.
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80.
Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–67.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–22.
Moore RE, Kawashiri MA, Kitajima K, Secreto A, Millar JS, Pratico D, et al. Apolipoprotein A-I deficiency results in markedly increased atherosclerosis in mice lacking the LDL receptor. Arterioscler Thromb Vasc Biol. 2003;23(10):1914–20.
Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100(17):1816–22.
Shah PK. Apolipoprotein A-I/HDL infusion therapy for plaque stabilization-regression: a novel therapeutic approach. Curr Pharm Des. 2007;13(10):1031–8.
Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13(6):433–44.
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35. This is the first study to demonstrate that cholesterol efflux capacity inversely correlated with carotid IMT and prevalent CAD, independently of HDL-C and apoA-I levels.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–93. This study demonstrated that baseline measure of cholesterol efflux capacity inversely correlated with incidence of cardiovascular events.
Nissen SE, Nicholls SJ, Wolski K, Howey DC, McErlean E, Wang MD, et al. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA. 2007;297(12):1362–73.
Lund EG, Menke JG, Sparrow CP. Liver X receptor agonists as potential therapeutic agents for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(7):1169–77.
McLure KG, Gesner EM, Tsujikawa L, Kharenko OA, Attwell S, Campeau E, et al. RVX-208, an inducer of ApoA-I in humans, is a BET bromodomain antagonist. PLoS One. 2013;8(12):e83190. This study demonstrated that RVX-208 binds to bromodomains of the BET family and affects apoA-I production via an epigenetic mechanism.
Fruchart JC. Selective peroxisome proliferator-activated receptor alpha modulators (SPPARMalpha): the next generation of peroxisome proliferator-activated receptor alpha-agonists. Cardiovasc Diabetol. 2013;12:82.
Singh JP, Kauffman R, Bensch W, Wang G, McClelland P, Bean J, et al. Identification of a novel selective peroxisome proliferator-activated receptor alpha agonist, 2-methyl-2-(4-{3-[1-(4-methylbenzyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-yl]prop yl}phenoxy)propanoic acid (LY518674), that produces marked changes in serum lipids and apolipoprotein A-1 expression. Mol Pharmacol. 2005;68(3):763–8.
Millar JS, Duffy D, Gadi R, Bloedon LT, Dunbar RL, Wolfe ML, et al. Potent and selective PPAR-alpha agonist LY518674 upregulates both ApoA-I production and catabolism in human subjects with the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2009;29(1):140–6.
Khera AV, Millar JS, Ruotolo G, Wang MD, Rader DJ. Potent peroxisome proliferator-activated receptor-alpha agonist treatment increases cholesterol efflux capacity in humans with the metabolic syndrome. Eur Heart J. 2015. doi:10.1093/eurheartj/ehv1291. This study shows that treatment with a PPAR-alpha agonist increase cholesterol efflux capacity, despite negligible changes in apoA-I and HDL-C levels, and that the increase in cholesterol efflux is positively correlated with the increase in apoA-I production.
Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, et al. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113(1):90–7.
Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors. LXRalpha LXRbeta Genes Dev. 2000;14(22):2819–30.
Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277(11):9520–8.
Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–31.
Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ Res. 2006;99(7):672–4.
Kirchgessner TG, Martin R, Sleph P, Grimm D, Liu X, Lupisella J, et al. Pharmacological characterization of a novel liver X receptor agonist with partial LXRalpha activity and a favorable window in nonhuman primates. J Pharmacol Exp Ther. 2015;352(2):305–14.
Giannarelli C, Cimmino G, Connolly TM, Ibanez B, Ruiz JM, Alique M, et al. Synergistic effect of liver X receptor activation and simvastatin on plaque regression and stabilization: an magnetic resonance imaging study in a model of advanced atherosclerosis. Eur Heart J. 2012;33(2):264–73.
Katz A, Udata C, Ott E, Hickey L, Burczynski ME, Burghart P, et al. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J Clin Pharmacol. 2009;49(6):643–9.
Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci U S A. 2013;110(49):19754–9. This study provides the potential mechanism of action of RVX-208 by its characterization as a selective bromodomain inhibitor, and demonstrates the feasibility of targeting a protein regulating transcription to affect apoA-I levels.
Jahagirdar R, Zhang H, Azhar S, Tobin J, Attwell S, Yu R, et al. A novel BET bromodomain inhibitor, RVX-208, shows reduction of atherosclerosis in hyperlipidemic ApoE deficient mice. Atherosclerosis. 2014;236(1):91–100.
Bailey D, Jahagirdar R, Gordon A, Hafiane A, Campbell S, Chatur S, et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010;55(23):2580–9.
Nicholls SJ, Gordon A, Johansson J, Wolski K, Ballantyne CM, Kastelein JJ, et al. Efficacy and safety of a novel oral inducer of apolipoprotein a-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J Am Coll Cardiol. 2011;57(9):1111–9.
Nicholls SJB, Barter PJ, Brewer HB, Kastelein JJP, Gordon A, Johansson J, Wong N, Puri R, Borgman M, Wolski K et al.: ASSURE: Effect of an oral agent inducing ApoA-I synthesis on progression of coronary atherosclerosis: Results of the ASSURE study. In: European Society of Cardiology Congress: 2013; Amsterdam; 2013.
Johansson JANO, Gordon AF CH, Wong NC. Effect of RVX-208 on major adverse cardiac events (MACE), apoprotein A-I and High-Density-Lipoprotein; A post-hoc analysis from the pooled SUSTAIN and ASSURE clinical trials. Eur Heart J. 2014;35(Abstract Supplement):723.
Siebel A, Trinh SK, Khan A, Johansson J, Allan G, Wong N, Otvos J, Rye KA, Barter P, Meikle P et al.: The effects of a novel apoA-I transcriptional regulator (RVX-208) on whole plasma and HDL lipidomes. In: 7th International Symposium on Atherosclerosis: 2015; Amsterdam, The Netherlands; 2015: A803.
Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Grijalva VR, et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 2004;109(25):3215–20.
Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, et al. Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002;105(3):290–2.
Navab M, Anantharamaiah GM, Reddy ST, Hama S, Hough G, Frank JS, et al. Oral small peptides render HDL antiinflammatory in mice and monkeys and reduce atherosclerosis in ApoE null mice. Circ Res. 2005;97(6):524–32.
Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103(18):2283–8.
Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008;49(6):1344–52.
Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011;52(2):361–73.
Reddy ST, Navab M, Anantharamaiah GM, Fogelman AM. Searching for a Successful HDL-based Treatment Strategy. Biochim Biophys Acta. 1841;2014:162–7.
Stoekenbroek RM, Stroes ES, Hovingh GK. ApoA-I mimetics. Handb Exp Pharmacol. 2015;224:631–48.
Kingwell BA, Chapman MJ, Kontush A, Miller NE. HDL-targeted therapies: progress, failure and future. Nat Rev Drug Discov. 2015;13:445–64.
Javaheri A, Kolansky DM, Cuchel M. Reconstituted high-density lipoprotein therapies: a cause for optimism. Arterioscler Thromb Vasc Biol. 2014;34:1800–2.
Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, Kapadia S, et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol. 2006;47(5):992–7.
Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007;297(15):1675–82.
Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103(10):1084–91.
Lerch PG, Fortsch V, Hodler G, Bolli R. Production and characterization of a reconstituted high density lipoprotein for therapeutic applications. Vox Sang. 1996;71(3):155–64.
Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, et al. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol. 2009;53(11):962–71.
Gille A, Easton R, D'Andrea D, Wright SD, Shear CL. CSL112 enhances biomarkers of reverse cholesterol transport after single and multiple infusions in healthy subjects. Arterioscler Thromb Vasc Biol. 2014;34(9):2106–14. This study shows that administration of CSL-112 in humans enhances the ability of the HDL to promote ex vivo cholesterol efflux.
Easton R, Gille A, D'Andrea D, Davis R, Wright SD, Shear C. A multiple ascending dose study of CSL112, an infused formulation of ApoA-I. J Clin Pharmacol. 2014;54(3):301–10.
Tricoci P, D'Andrea DM, Gurbel PA, Yao Z, Cuchel M, Winston B, et al. Infusion of reconstituted high-density lipoprotein, CSL112, in patients with atherosclerosis: safety and pharmacokinetic results from a phase 2a randomized clinical trial. J Am Heart Assoc. 2015;4(8):e002171. doi:10.1161/JAHA.115.002171.
Roma P, Gregg RE, Meng MS, Ronan R, Zech LA, Franceschini G, et al. In vivo metabolism of a mutant form of apolipoprotein A-I, apo A-IMilano, associated with familial hypoalphalipoproteinemia. J Clin Invest. 1993;91(4):1445–52.
Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, et al. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103(25):3047–50.
Parolini C, Marchesi M, Lorenzon P, Castano M, Balconi E, Miragoli L, et al. Dose-related effects of repeated ETC.-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008;51(11):1098–103.
Ibanez B, Vilahur G, Cimmino G, Speidl WS, Pinero A, Choi BG, et al. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I Milano (ETC.-216) administration: magnetic resonance imaging study in an experimental model of atherosclerosis. J Am Coll Cardiol. 2008;51(11):1104–9.
Ibanez B, Giannarelli C, Cimmino G, Santos-Gallego CG, Alique M, Pinero A, et al. Recombinant HDL(Milano) exerts greater anti-inflammatory and plaque stabilizing properties than HDL(wild-type). Atherosclerosis. 2012;220(1):72–7.
Giannarelli C, Cimmino G, Ibanez B, Chiesa G, Garcia-Prieto J, Santos-Gallego CG, et al. Acute ApoA-I Milano administration induces plaque regression and stabilisation in the long term. Thromb Haemost. 2012;108(6):1246–8.
Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290(17):2292–300.
Kempen HJ, Schranz DB, Asztalos BF, Otvos J, Jeyarajah E, Drazul-Schrader D, et al. Incubation of MDCO-216 (ApoA-IMilano/POPC) with Human Serum Potentiates ABCA1-Mediated Cholesterol Efflux Capacity, Generates New Prebeta-1 HDL, and Causes an Increase in HDL Size. J Lipids. 2014;2014:923903.
Kempen H, Kallend D, Bobillier A, Eralp B, Wijngaard PL, Asztalos B: Cholesterol efflux capacity and HDL subfractions after a single ascending dose of MDCO-216 (apoAI-Milano/POPC) in humanvolunteers and stable CAD patients. In: 7th International Symposium on Atherosclerosis: 2015; Amsterdam, The Netherlands; 2015: A850.
Kallend DG, Bobillier A, Belibas SE, Kempen H, Burggraaf J, Reijers J, et al. A Single Infusion of MDCO-216 (ApoA-1 Milano/POPC) Induces Marked Changes on the Lipid Profile. Circulation. 2014;130:A9907.
Bellibas SE, Kallend D, Bobillier A, Kempen H, Wijngaard PL. Single Ascending Dose Pharmacokinetics and Pharmacodynamics of MDCO-216 (ApoA-I Milano/POPC) in Healthy Volunteers. Circulation. 2014;130:A13324.
Sacks FM, Rudel LL, Conner A, Akeefe H, Kostner G, Baki T, et al. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J Lipid Res. 2009;50(5):894–907.
Waksman R, Torguson R, Kent KM, Pichard AD, Suddath WO, Satler LF, et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J Am Coll Cardiol. 2010;55(24):2727–35.
Tardy C, Goffinet M, Boubekeur N, Ackermann R, Sy G, Bluteau A, et al. CER-001, a HDL-mimetic, stimulates the reverse lipid transport and atherosclerosis regression in high cholesterol diet-fed LDL-receptor deficient mice. Atherosclerosis. 2014;232(1):110–8.
Tardif JC, Ballantyne CM, Barter P, Dasseux JL, Fayad ZA, Guertin MC, et al. Effects of the high-density lipoprotein mimetic agent CER-001 on coronary atherosclerosis in patients with acute coronary syndromes: a randomized trial. Eur Heart J. 2014;35(46):3277–86.
Hovingh GK, Smits LP, Stefanutti C, Soran H, Kwok S, de Graaf J, et al. The effect of an apolipoprotein A-I-containing high-density lipoprotein-mimetic particle (CER-001) on carotid artery wall thickness in patients with homozygous familial hypercholesterolemia: The Modifying Orphan Disease Evaluation (MODE) study. Am Heart J. 2015;169(5):736–742 e731. This is a proof of principle study suggesting that treatment with CER-001 my affects atherosclerotic burden in subjects with homozygous familial hypercholesterolemia on maximal lipid-lowering treatment.
Kootte RS, Smits LP, van der Valk FM, Dasseux JL, Keyserling CH, Barbaras R et al.: Effect of open-label infusion of an apolipoprotein A-I-containing particle (CER-001) on reverse cholesterol transport and artery wall thickness in patients with familial hypo-alphalipoproteinemia. J Lipid Res 2015. This is a proof of principle study suggesting that treatment with CER-001 not only enhance ex vivo cholesterol efflux capacity, but also affects atherosclerotic burden and inflammation in subjects with familial hypoalphalipoproteinemia.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
John S. Millar has received grants from Merck and Eli Lilly as well as honoraria payments from Merck.
Marina Cuchel has received grants from CSL Behring, Sanofi, Regeneron, and Aegerion Pharmaceuticals as well as honoraria payments and paid travel accommodations from Aegerion Pharmaceuticals.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Nonstatin Drugs
Rights and permissions
About this article
Cite this article
Millar, J.S., Cuchel, M. ApoA-I-Directed Therapies for the Management of Atherosclerosis. Curr Atheroscler Rep 17, 60 (2015). https://doi.org/10.1007/s11883-015-0539-0
Published:
DOI: https://doi.org/10.1007/s11883-015-0539-0