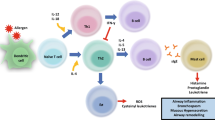
Abstract
House dust mites (HDMs) are found in the environments where human habitation exists. Their density is dependent on environmental relative humidity; therefore, higher populations are present in areas of the world with higher humidity levels, e.g., coastal areas and tropics. To date, 24 HDM allergens have been identified. Many of these represent digestive enzymes since HDM feces are the major source of allergen exposure. IgE- medicated sensitization to HDM allergens is an important factor in the pathogenesis of allergic diseases since it is the most common aeroallergen detected by skin testing or in vitro IgE assays. Sensitization to HDM allergens often occurs early in life and appears to play an important role in the progression from allergic rhinitis to asthma (the so-called Allergic March) in children. HDM sensitization is also associated with asthma across all age groups. Efforts to control environmental exposure to HDM allergens have often proven to be unsuccessful. While medications can improve symptoms, only immunotherapy currently provides disease-modifying effects in allergic rhinitis and asthma. Several systemic reviews and meta-analysis indicate that both subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT) are effective in the treatment of allergic rhinitis and asthma for HDM sensitivity. In this report, we review recent studies and the evidence for the use of HDM SCIT and SLIT. Fundamental gaps in knowledge are identified which could lead to improved approaches to HDM allergy.
Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- Der p 1, Der p 2, Der p 23:
-
House dust mite Dermatophagoides pteronyssinus allergens
- Der f 1, Der f 2:
-
House dust mite Dermatophagoides farinae allergens
- HDM:
-
House dust mites
- IgA2, IgG4:
-
Immunoglobulin, A2 and G4
- IL-10:
-
Interleukin-10
- NNT:
-
Number needed to treat
- RR:
-
Relative risk ratio
- SCIT:
-
Subcutaneous immunotherapy
- SLIT:
-
Sublingual immunotherapy
- SMD:
-
Standardized mean difference
- TGF-beta:
-
Transforming growth factor beta
- TH1, TH2:
-
T helper cells, type 1 and 2
- Tregs:
-
T regulatory Cells
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Portnoy J, Miller JD, Williams PB, Chew GL, Miller JD, Zaitoun F, et al. Environmental assessment and exposure control of dust mites: a practice parameter. Ann Allergy Asthma Immunol. 2013;111(6):465–507.
Blythe ME, Williams JD, Smith JM. Distribution of pyroglyphid mites in Birmingham with particular reference to Euroglyphouse maynei. Clin Allergy. 1974;4(1):25–33.
Colloff M. Dust mites. Collingwood: Springer; 2009.
Platts-Mills TAE, Indoor Allergens. In: Adkinson NF Jr, Bochner BS, Burks AW, Busse WW, Holgate ST, Lemanske RF Jr, O'Herir RE, editors. Middleton's allergy: principles and practice. 8th ed, 2014. p. 455–458.
Voorhorst R, Spieksma FTM, Varekamp H, Leupen MJ, Lyklema AW. The house-dust mite (Dermatophagoides pteronyssinus) and the allergens it produces. Identity with the house-dust allergen. J Allergy. 1967;39(6):325–39.
Weghofer M, Grote M, Resch Y, Casset A, Kneidinger M, Kopec J, et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol. 2013;190(7):3059–67.
Banerjee S, Resch Y, Chen KW, Swoboda I, Focke-Tejkl M, Blatt K, Novak N, van Hage M, Ferrara R, Mari A, Purohit A, Pauli G, Sibanda EN, Ndlovu P, Thomas WR, Krzyzanek V, Tacke S, Malkus U, Valent P, Valenta R, Vrtala S Banerjee, Der p 11 is a major allergen for house dust mite allergic patients suffering from atopic dermatitis. J Invest Dermatol. 2014.
Pevec MR, Markovic AS, Batista I. House dust mite subcutaneous immunotherapy does not induce new sensitization to tropomyosin: does it do the opposite? J investig Allergol Clin Immunol. 2014;24(1):29–34.
Larenas-Linnemann D, Michels A, Dinger H, Shah-Hosseini K, Mösges R, Arias-Cruz A, et al. Allergen sensitization linked to climate and age, not to intermittent-persistent rhinitis in a cross-sectional cohort study in the (sub)tropics. Clin Transl Allergy. 2014;4:20.
Dust mite allergens and asthma: a worldwide problem. International Workshop report. Bull World Health Organ. 1988; 66(6): 769–80. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2491145/.
Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ, et al. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323(8):502–7. An important study relating exposure to HDM allergens and the development of asthma in children.
Sears MR, Herbison GP, Holdaway MD, Hewitt CJ, Flannery EM, Silva PA. The relative risks of sensitivity to grass pollen, house dust mite and cat dander in the development of childhood asthma. Clin Exp Allergy. 1989;19(4):419–24.
Jaén A, Sunyer J, Basagaña X, Chinn S, Zock JP, Antó JM, et al. Specific sensitization to common allergens and pulmonary function in the European Community Respiratory Health Survey. Clin Exp Allergy. 2002;32(12):1713–9.
Corzo JL, Carrillo T, Pedemonte C, Plaza Martin AM, Martín Hurtado S, Dige E, et al. Tolerability during double-blind randomized phase I trials with the house dust mite allergy immunotherapy tablet in adults and children. J Investig Allergol Clin Immunol. 2014;24(3):154–61.
Linneberg A, Henrik Nielsen N, Frølund L, Madsen F, Dirksen A, Jørgensen T, et al. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002;57(11):1048–52.
Kim J, Lee S, Woo SY, Han Y, Lee JH, Lee IY, et al. The indoor level of house dust mite allergen is associated with severity of atopic dermatitis in children. J Korean Med Sci. 2013;28(1):74–9.
Nurmatov U, van Schayck CP, Hurwitz B, Nurmatov SA. House dust mite avoidance measures for perennial allergic rhinitis: an updated Cochrane systematic review. Allergy. 2012;67(2):158–65.
Wright LS, Phipatanakul W. Environmental remediation in the treatment of allergy and asthma: latest updates. Curr Allergy Asthma Rep. 2014;14(3):419. A review of current environmental control practices for HDM allergens.
Pilette C, Nouri-Aria KT, Jacobson MR, Wilcock LK, Detry B, Walker SM, et al. Grass-pollen immunotherapy induces an allergen-specific IgA2 antibody response associated with mucosal TGF-beta expression. J Immunol. 2007;178:4658–66.
Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14.
Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy. 2011;41:1235–46.
Pifferi M, Badlini G, Marrazzini G, Baldini M, Ragazzo V, Pietrobelli A, et al. Benefits of immunotherapy with a standardized Dermatophagoides pteronyssinus extract in asthmatic children: a three-year prospective study. Allergy. 2002;57:785–90.
Varny VA, Tabbah K, Mavroleon G, Frew AJ. Usefulness of specific immunotherapy in patients with severe perennial allergic rhinitis induced by house dust mite: a double-blind, randomized, placebo-controlled trial. Clin Exp Allergy. 2003;33:1076–82.
Abramson MJ, Puy RM, Winer JM. Injection allergen immunotherapy for asthma. Database Syst Rev. 2010;8, CD001186. Reviews the role of allergen immunotherapy in asthma.
Calderon MA, Alves B, Jacbonson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;1, CD001936. Reviews the role of allergen immunotherapy for allergic rhinitis.
Calderon MA, Casale TB, Nelson HS, Demoly P. An evidence-based analysis of house dust mite allergen immunotherapy: a call for more rigorous clinical studies. J Allergy Clin Immunol. 2013;1320:1322–36. A seminal review of HDM immunotherapy.
Pajno GB, Barberio G, De Luca F, et al. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy. A six-year follow-up study. Clin Exp Allergy. 2001;31:1392–7.
Des Roches A, Paradis L, Menardo JL, Bouges S, Daurés JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI. Specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997;99:450–3. HDM immunotherapy may prevent “The Allergic March”.
Nieto García A, Nevot Falcó S, Carriollo Díaz T, Cumplido Bonny JA, Izquierdo Calderón JP, Hernández-Peña J. Safety of cluster specific immunotherapy with a modified high-dose house dust mite extract. Eur Ann Allergy Clin Immunol. 2013;45:78–83.
Vrtala S, Huber H, Thomas WR. Recombinant house dust mite allergens. Methods. 2014;66:67–74.
Asturias JA, Ibarrola I, Arilla MC, Vidal C, Ferrer A, Gamboa PM, et al. Engineering of major house dust mite allergens Der p1 and Der p 2 for allergen-specific immunotherapy. Clin Exp Allergy. 2009;39:1088–98.
Baris S, Kiykim A, Ozen A, Tulunay A, Karakoc-Aydiner E, Barlan IB. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy. 2014;69:246–53.
Bush RK, Swenson C, Fahlberg B, Evans MD, Esch R, Morris M, et al. House dust mite sublingual immunotherapy: results of a US trial. J Allergy Clin Immunol. 2011;127:974–81. el-7.
Van Dyken AM, Smith PK, Fox TL. Clinical case of anaphylaxis with sublingual immunotherapy: house dust mite allergen. J Allergy Clin Immunol: Pract. 2014;2:485–6.
Calderon MA, Simons FER, Malling HJ, Cockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy. 2007;62:567–8.
Bozek A, Ignasiak B, Filiipowska B, et al. House dust mite sublingual immunotherapy: a double-blind, placebo-controlled study in elderly patients with allergic rhinitis. Clin Exp Allergy. 2013;43:242–8.
Wang DH, Chen L, Cheng L, et al. Fast onset of action of sublingual immunotherapy in house dust mite-induced allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial. Laryngoscope. 2013;123:1334–40.
Querios MGJ, Silva DAO, Siman IL, et al. Modulation of mucosal/systemic antibody response after sublingual immunotherapy in mite-allergic children. Ped Allergy Immunol. 2013;24:752–61.
Tian M, Wang Y, Lu Y, et al. Effects of sublingual immunotherapy for Dermatophagoides farina on Th17 cells and CD4+ CD25+ regulatory T cells in peripheral blood of children with allergic asthma. Int Forum Allergy Rhinol. 2014; Article first published online: 3 MAR 2014.
Bergmann K-C, Demoly P, Worm M, Fokkens WJ, Carrillo T, Tabar AI, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. 2014;133:1608–14.
Erekosima N, Suarez-Cuervo C, Rammanathan M, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope. 2014;124:616–27.
Lin SY, Erekosima N, Kim JM, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013;309:1278–88.
Nelson HS. New forms of allergy immunotherapy for rhinitis and asthma. Allergy Asthma Proc. 2014;35:271–7.
Pajno GB, Caminiti L, Vita D, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. 2007;120:164–70.
Compliance with Ethics Guidelines
Conflict of Interest
Robert Bush is an Associate Editor of the Journal of Allergy and Clinical Immunology. He also serves as a Section Editor for Current Allergy and Asthma Reports and Current Opinions in Allergy and Clinical Immunology. Mark Biagtan and Ravi Viswanathan report no disclosures.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Immunotherapy and Immunomodulators
Rights and permissions
About this article
Cite this article
Biagtan, M., Viswanathan, R. & Bush, R.K. Immunotherapy for House Dust Mite Sensitivity: Where Are the Knowledge Gaps?. Curr Allergy Asthma Rep 14, 482 (2014). https://doi.org/10.1007/s11882-014-0482-0
Published:
DOI: https://doi.org/10.1007/s11882-014-0482-0