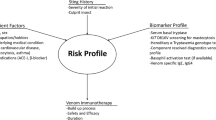
Abstract
Venom immunotherapy (VIT) is the most effective form of specific immunotherapy to date. Hitherto, several relevant queries remain unanswered, namely optimal doses, duration, and means of assessment. Important progress has been lately made in terms of diagnosis by means of component-resolved diagnosis. Moreover, basophil activation test results in patients with negative serum immunoglobulin E (IgE) and skin prick test confer this technique a promising future, although these outcomes shall be considered with caution. This review aims to unravel the important advances made on diagnosis, management, and prognosis and also focuses on several undetermined aspects of VIT.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Golden DB. Advances in diagnosis and management of insect sting allergy. Ann Allergy Asthma Immunol. 2013;111(2):84–9. Summarizes recent advances in the field of venom immunotherapy and raises some unmet needs which require further investigation.
Aberer W, Sturm G. Venom immunotherapy: pitfalls and open questions. Immunotherapy. 2011;3(11):1277–9. Discusses important aspects about insect hypersensitivity and states open questions which remain unanswered.
Papadopoulos NG, Agache I, Bavbek S, Bilo BM, Braido F, Cardona V, et al. Research needs in allergy: an EAACI position paper, in collaboration with EFA. Clin Transl Allergy. 2012;2(1):21.
Golden DB. Discontinuing venom immunotherapy. Curr Opin Allergy Clin Immunol. 2001;1(4):353–6.
Burks AW, Calderon MA, Casale T, Cox L, Demoly P, Jutel M, et al. Update on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2013;131(5):1288–96.e3. This international consensus report on immunotherapy reviews mechanisms of AIT and its use in clinical practice, as well as unmet needs and ongoing developments in AIT.
Cox L, Compalati E, Kundig T, Larche M. New directions in immunotherapy. Curr Allergy Asthma Rep. 2013;13(2):178–95. New means of immunotherapy are devised, namely oral immunotherapy, intralymphatic immunotherapy and epicutaneous immunotherapy. Other aspects as vaccine modifications, by means of T cell epitopes or the use of viral-like particles are covered.
Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1–55. Contraindications, risk factors, comorbidities, doses, and routes of administration are examinated in this practice parameter.
Goldberg A, Confino-Cohen R. Bee venom immunotherapy—how early is it effective? Allergy. 2010;65(3):391–5.
Oude Elberink JN, De Monchy JG, Van Der Heide S, Guyatt GH, Dubois AE. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. J Allergy Clin Immunol. 2002;110(1):174–82.
Oude Elberink JN, de Monchy JG, Golden DB, Brouwer JL, Guyatt GH, Dubois AE. Development and validation of a health-related quality-of-life questionnaire in patients with yellow jacket allergy. J Allergy Clin Immunol. 2002;109(1):162–70.
Calderón MA, Larenas D, Kleine-Tebbe J, Jacobsen L, Passalacqua G, Eng PA, et al. European Academy of Allergy and Clinical Immunology task force report on ‘dose-response relationship in allergen-specific immunotherapy’. Allergy. 2011;66(10):1345–59. It evaluates the currently available data on dose-response relationships in SIT and aims to provide recommendations for the design of future studies.
Calderon MA, Demoly P, Gerth van Wijk R, Bousquet J, Sheikh A, Frew A, et al. EAACI: a European declaration on immunotherapy. Designing the future of allergen specific immunotherapy. Clin Transl Allergy. 2012;2(1):20.
Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E, et al. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61 Suppl 82:1–20.
Bonifazi F, Jutel M, Biló BM, Birnbaum J, Muller U. EAACI Interest Group on Insect Venom Hypersensitivity. Prevention and treatment of hymenoptera venom allergy: guidelines for clinical practice. Allergy. 2005;60(12):1459–70.
Biló BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN. EAACI Interest Group on Insect Venom Hypersensitivity. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60(11):1339–49.
Strohmeier B, Aberer W, Bokanovic D, Komericki P, Sturm GJ. Simultaneous intradermal testing with hymenoptera venoms is safe and more efficient than sequential testing. Allergy. 2013;68(4):542–4. It supports promising data due to the optimization of time required to diagnose a patient who is allergic to hymenoptera venoms. Challenges current procedures and suggests a new approach in this kind of patients.
Niedoszytko M, Grucha A-Niedoszytko M, Jassem E. Gene expression analysis in allergology: the prediction of Hymenoptera venom allergy severity and treatment efficacy. Clin Transl Allergy. 2013;3(1):35.
Niedoszytko M, Bruinenberg M, de Monchy J, Weersma RK, Wijmenga C, Jassem E, et al. Changes in gene expression caused by insect venom immunotherapy responsible for the long-term protection of insect venom-allergic patients. Ann Allergy Asthma Immunol. 2011;106(6):502–10.
Müller U, Schmid-Grendelmeier P, Hausmann O, Helbling A. IgE to recombinant allergens Api m 1, Ves v 1, and Ves v 5 distinguish double sensitization from crossreaction in venom allergy. Allergy. 2012;67(8):1069–73. Component resolved diagnosis of insect venom allergy is unraveled and raises the importance of its accuracy to achieve improved results with VIT.
Monsalve RI, Vega A, Marqués L, Miranda A, Fernández J, Soriano V, et al. Component-resolved diagnosis of vespid venom-allergic individuals: phospholipases and antigen 5s are necessary to identify Vespula or Polistes sensitization. Allergy. 2012;67(4):528–36. This article establishes the importance of phoshpholipases and antigen 5s, confirmed by CRD to discriminate the probable sensitizing species in vespid-allergic patients.
Treudler R, Simon JC. Overview of component resolved diagnostics. Curr Allergy Asthma Rep. 2013;13(1):110–7. Updates current developments of CRD, including multiarray test systems in order to distinguish between clinically significant and irrelevant sIgE results, aiming to improve prognosis.
Incorvaia C, Mauro M. Can component-resolved diagnosis overturn the current knowledge on vespid allergy? Allergy. 2012;67(7):966.
Vos B, Köhler J, Müller S, Stretz E, Ruëff F, Jakob T. Spiking venom with rVes v 5 improves sensitivity of IgE detection in patients with allergy to Vespula venom. J Allergy Clin Immunol. 2013;131(4):1225–7. 1227.e1.
Korošec P, Valenta R, Mittermann I, Celesnik N, Eržen R, Zidarn M, et al. Low sensitivity of commercially available rApi m 1 for diagnosis of honeybee venom allergy. J Allergy Clin Immunol. 2011;128(3):671–3.
Sturm GJ, Biló MB, Bonadonna P, Hemmer W, Caruso B, Bokanovic D et al. Ves v 5 can establish the diagnosis in patients without detectable specific IgE to wasp venom and a possible north-south difference in Api m 1 sensitization in Europe. J Allergy Clin Immunol 2012;130(3):817; author reply 818–9. Regional differences may appear in patients suffering from insect venom allergy.
Blank S, Seismann H, Michel Y, McIntyre M, Cifuentes L, Braren I, et al. Api m 10, a genuine A. mellifera venom allergen, is clinically relevant but underrepresented in therapeutic extracts. Allergy. 2011;66(10):1322–9. This article delves into inquiries regarding the specific allergens that VIT may contain depending on patient sensitization profile.
Michel Y, McIntyre M, Ginglinger H, Ollert M, Cifuentes L, Blank S, et al. The putative serine protease inhibitor Api m 6 from Apis mellifera venom: recombinant and structural evaluation. J Investig Allergol Clin Immunol. 2012;22(7):476–84.
Köhler J, Blank S, Müller S, Bantleon F, Frick M, Huss-Marp J et al. Component resolution reveals additional major allergens in patients with honeybee venom allergy. J Allergy Clin Immunol 2014;133(5):1383–1389.e6
Bilò MB. Anaphylaxis caused by Hymenoptera stings: from epidemiology to treatment. Allergy. 2011;66 Suppl 95:35–7. The article reviews the epidemiology, current strategies for reducing adverse reactions, differences in extracts in terms of components, as well as new strategies for VIT.
Ruëff F, Przybilla B, Biló MB, Müller U, Scheipl F, Aberer W, et al. Predictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: the importance of baseline serum tryptase. J Allergy Clin Immunol. 2010;126(1):105–11.e5.
Korošec P, Valenta R, Mittermann I, Celesnik N, Silar M, Zidarn M, et al. High sensitivity of CAP-FEIA rVes v 5 and rVes v 1 for diagnosis of Vespula venom allergy. J Allergy Clin Immunol. 2012;129(5):1406–8.
Sanz ML, Gamboa PM, De Weck AL. In vitro tests: basophil activation tests. In: Pichler WJ, editor. Drug hypersensitivity. Basel: Karger; 2007. p. 391–402.
Korošec P, Šilar M, Eržen R, Čelesnik N, Bajrović N, Zidarn M, et al. Clinical routine utility of basophil activation testing for diagnosis of hymenoptera-allergic patients with emphasis on individuals with negative venom-specific IgE antibodies. Int Arch Allergy Immunol. 2013;161(4):363–8.
Korosec P, Erzen R, Silar M, Bajrovic N, Kopac P, Kosnik M. Basophil responsiveness in patients with insect sting allergies and negative venom-specific immunoglobulin E and skin prick test results. Clin Exp Allergy. 2009;39(11):1730–7.
Eberlein B, Krischan L, Darsow U, Ollert M, Ring J. Double positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2012;130(1):155–61.
Bonadonna P, Zanotti R, Melioli G, Antonini F, Romano I, Lenzi L, et al. The role of basophil activation test in special populations with mastocytosis and reactions to hymenoptera sting. Allergy. 2012;67(7):962–5. Basophil Activation Test may confirm negative results from intradermal tests in mastocytosis patients who had suffered reactions to hymenoptera.
González-de-Olano D, Alvarez-Twose I, Morgado JM, Esteban López MI, Vega Castro A, Díaz de Durana MD, et al. Evaluation of basophil activation in mastocytosis with Hymenoptera venom anaphylaxis. Cytometry B Clin Cytom. 2011;80(3):167–75.
Peternelj A, Silar M, Erzen R, Kosnik M, Korosec P. Basophil sensitivity in patients not responding to venom immunotherapy. Int Arch Allergy Immunol. 2008;146(3):248–54.
Žitnik SE, Vesel T, Avčin T, Šilar M, Košnik M, Korošec P. Monitoring honeybee venom immunotherapy in children with the basophil activation test. Pediatr Allergy Immunol. 2012;23(2):166–72.
Boyle RJ, Elremeli M, Hockenhull J, Cherry MG, Bulsara MK, Daniels M, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012;10, CD008838. Meta-analysis which comprises evidence on venom immunotherapy. 6 randomised controlled trials and 1 quasi-randomised controlled trial were included in the review.
Ruëff F, Przybilla B,Müller U,Mosbech H. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996;51(4):216–25.
Cortellini G, Severino M, Francescato E, Turillazzi S, Spadolini I, Rogkakou A, et al. Evaluation and validation of a bee venom sting challenge performed by a micro-syringe. Ann Allergy Asthma Immunol. 2012;109(6):438–41.
Golden DB, Breisch NL, Hamilton RG, Guralnick MW, Greene A, Craig TJ, et al. Clinical and entomological factors influence the outcome of sting challenge studies. J Allergy Clin Immunol. 2006;117(3):670–5.
Carballada F, Boquete M, Núñez R, Lombardero M, de la Torre F. Follow-up of venom immunotherapy (VIT) based on conventional techniques and monitoring of immunoglobulin E to individual venom allergens. J Investig Allergol Clin Immunol. 2010;20(6):506–13.
Golden DB, Marsh DG, Freidhoff LR, Kwiterovich KA, Addison B, Kagey-Sobotka A, et al. Natural history of Hymenoptera venom sensitivity in adults. J Allergy Clin Immunol. 1997;100(6 Pt 1):760–6.
Goldberg A, Confino-Cohen R. Rush venom immunotherapy in patients experiencing recurrent systemic reactions to conventional venom immunotherapy. Ann Allergy Asthma Immunol. 2003;91(4):405–10.
Riccio AM, Saverino D, Pesce G, Rogkakou A, Severino M, Bonadonna P, et al. Effects of different up-dosing regimens for hymenoptera venom immunotherapy on serum CTLA-4 and IL-10. PLoS One. 2012;7(6):e37980. Means of assessment are one of the pitfalls with regards immunotherapy. This article demonstrates changes in markers after using different initiation schedules.
Čelesnik N, Vesel T, Rijavec M, Šilar M, Eržen R, Košnik M, et al. Short-term venom immunotherapy induces desensitization of FcεRI-mediated basophil response. Allergy. 2012;67(12):1594–600.
Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010;65(12):1558–65.
Novak N, Mete N, Bussmann C, Maintz L, Bieber T, Akdis M, et al. Early suppression of basophil activation during allergen-specific immunotherapy by histamine receptor 2. J Allergy Clin Immunol. 2012;130(5):1153–1158.e2.
Jutel M, Akdis CA. Immunological mechanisms of allergen-specific immunotherapy. Allergy. 2011;66(6):725–32.
Pierkes M, Bellinghausen I, Hultsch T, Metz G, Knop J, Saloga J. Decreased release of histamine and sulfidoleukotrienes by human peripheral blood leukocytes after wasp venom immunotherapy is partially due to induction of IL-10 and IFN-gamma production of T cells. J Allergy Clin Immunol. 1999;103(2 Pt 1):326–32.
Müller U, Helbling A, Bischof M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy. 1989;44(6):412–8.
Ruëff F, Przybilla B, Biló MB, Müller U, Scheipl F, Seitz MJ, et al. Clinical effectiveness of hymenoptera venom immunotherapy: a prospective observational multicenter study of the European academy of allergology and clinical immunology interest group on insect venom hypersensitivity. PLoS One. 2013;8(5):e63233. Follow-up of 357 patients with established honey bee or vespid venom allergy after the maintenance dose of VIT had been reached. The most important factor associated with VIT failure was a honey bee venom allergy. Preventive use of anti-allergic drugs may be associated with a higher protection rate.
Sánchez-Machín I, Moreno C, González R, Iglesias-Souto J, Pérez E, Matheu V. Safety of a 2-visit cluster schedule of venom immunotherapy in outpatients at risk of life-threatening anaphylaxis. J Investig Allergol Clin Immunol. 2010;20(1):91–2.
Moreno C, Barasona MJ, Serrano P, Justicia JL, Ruz JM, Guerra F. Alternating Polistes-Vespula venom immunotherapy: a therapeutic strategy to resolve a diagnostic deficiency. J Investig Allergol Clin Immunol. 2011;21(1):28–33.
Bilò MB, Cinti B, Brianzoni MF, Braschi MC, Bonifazi M, Antonicelli L. Honeybee venom immunotherapy: a comparative study using purified and nonpurified aqueous extracts in patients with normal Basal serum tryptase concentrations. J Allergy (Cairo). 2012;2012:869243.
Ruëff F, Wenderoth A, Przybilla B. Patients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol. 2001;108(6):1027–32.
Konstantinou GN, Manoussakis E, Douladiris N, Hatziioannou A, Giavi S, Saxoni-Papageorgiou P, et al. A 5-year venom immunotherapy protocol with 50 μg maintenance dose: safety and efficacy in school children. Pediatr Allergy Immunol. 2011;22(4):393–7.
Golden DB, Kagey-Sobotka A, Valentine MD, Lichtenstein LM. Prolonged maintenance interval in hymenoptera venom immunotherapy. J Allergy Clin Immunol. 1981;67(6):482–4.
Goldberg A, Confino-Cohen R. Maintenance venom immunotherapy administered at 3-month intervals is both safe and efficacious. J Allergy Clin Immunol. 2001;107(5):902–6.
Cavallucci E, Ramondo S, Renzetti A, Turi MC, Di Claudio F, Braga M, et al. Maintenance venom immunotherapy administered at a 3-month interval preserves safety and efficacy and improves adherence. J Investig Allergol Clin Immunol. 2010;20(1):63–8.
Simioni L, Vianello A, Bonadonna P, Marcer G, Severino M, Pagani M, et al. Efficacy of venom immunotherapy given every 3 or 4 months: a prospective comparison with the conventional regimen. Ann Allergy Asthma Immunol. 2013;110(1):51–4. Currently, intervals of venom immunotherapy on maintenance dose range from 1–2 months. The authors performed a prospective study in seventy-six patients. The percentage of re-sting without reaction was 93.5 % in the extended maintenance dose group and 81.5 % in the conventionan maintenance dose group.
Bousquet J, Müller UR, Dreborg S, Jarisch R, Malling HJ, Mosbech H, et al. Immunotherapy with hymenoptera venoms. Position paper of the Working Group on Immunotherapy of the European Academy of Allergy and Clinical Immunology. Allergy. 1987;42(6):401–13.
Müller U. Epidemiology of insect sting allergy. Monogr Allergy. 1993;31:131–46.
Mosbech H, Müller U. Side-effects of insect venom immunotherapy: results from an EAACI multicenter study. Eur Acad Allergol Clin Immunol Allergy. 2000;55(11):1005–10.
Brockow K, Kiehn M, Riethmüller C, Vieluf D, Berger J, Ring J. Efficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: a prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol. 1997;100(4):458–63.
Gorska L, Chelminska M, Kuziemski K, Skrzypski M, Niedoszytko M, Damps-Konstanska I, et al. Analysis of safety, risk factors and pretreatment methods during rush hymenoptera venom immunotherapy. Int Arch Allergy Immunol. 2008;147(3):241–5.
Bilò MB, Antonicelli L, Bonifazi F. Purified vs. nonpurified venom immunotherapy. Curr Opin Allergy Clin Immunol. 2010;10(4):330–6.
Seppälä U, Francese S, Turillazzi S, Moneti G, Clench M, Barber D. In situ imaging of honeybee (Apis mellifera) venom components from aqueous and aluminum hydroxide-adsorbed venom immunotherapy preparations. J Allergy Clin Immunol. 2012;129(5):1314–1320.e3.
González-de-Olano D, Alvarez-Twose I, Vega A, Orfao A, Escribano L. Venom immunotherapy in patients with mastocytosis and hymenoptera venom anaphylaxis. Immunotherapy. 2011;3(5):637–51. Severe adverse reactions tohymenoptera stings or venom immunotherapy have been associated with increased serum baseline tryptase; however, presence of clonal MC has not been ruled out in most reports and thus both SM and clonal MC activation syndrome might be underdiagnosed in such patients. A new clinical score for such patients is advised.
Alvarez-Twose I, González-de-Olano D, Sánchez-Muñoz L, Matito A, Jara-Acevedo M, Teodosio C, et al. Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms. Int Arch Allergy Immunol. 2012;157(3):275–80.
Alvarez-Twose I, Bonadonna P, Matito A, Zanotti R, González-de-Olano D, Sánchez-Muñoz L, et al. Systemic mastocytosis as a risk factor for severe Hymenoptera sting-induced anaphylaxis. J Allergy Clin Immunol. 2013;131(2):614–5.
Alvarez-Twose I, Zanotti R, González-de-Olano D, Bonadonna P, Vega A, Matito A et al. Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J Allergy Clin Immunol. 2013 Aug 3. Indolent systemic mastocytosis (ISM) without skin lesions (ISMs − ) patients with anaphylaxis triggered exclusively by insects display clinical and laboratory features significantly different from other ISM cases with cutaneous lesions, such as male predominance, lower serum baseline tryptase levels, and KIT mutation more frequently restricted to bone marrow (BM) mast cells (MCs).
Golden DB. Long-term outcome after venom immunotherapy. Curr Opin Allergy Clin Immunol. 2010;10(4):337–41.
Compliance with Ethics Guidelines
Conflict of Interest
Darío Antolín-Amérigo and Melchor Alvarez-Mon are partially supported by research grant 2502 MITIC-CM from Comunidad de Madrid. Carmen Moreno Aguilar declares that she has no conflict of interest. Arantza Vega has received payment for development of educational presentations (include service on speakers bureaus) from Laboratorios Leti.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Immunotherapy and Immunomodulators
Rights and permissions
About this article
Cite this article
Antolín-Amérigo, D., Moreno Aguilar, C., Vega, A. et al. Venom Immunotherapy: an Updated Review. Curr Allergy Asthma Rep 14, 449 (2014). https://doi.org/10.1007/s11882-014-0449-1
Published:
DOI: https://doi.org/10.1007/s11882-014-0449-1