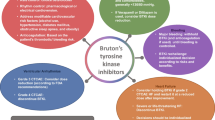
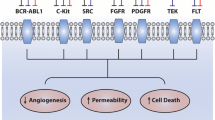
Opinion statement
There has been a significant shift in the management of B cell malignancies over the past decade. Initial strategies involving the use of systemic chemotherapies have been gradually replaced by more targeted therapies to improve survival and overall tolerability. Bruton’s tyrosine kinase inhibitors are breakthrough drugs that have been approved to treat many B cell malignancies. Despite their demonstrated benefits, unintended events still occur including various cardiotoxicities. In this review, we discuss the rationale behind developing these agents, their common cardiovascular toxicities, and associated management challenges.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists’ Collaborative Group. J Natl Cancer Inst. 1999;91:861–8.
Hallek M. Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017;92:946–65.
Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114:800–9.
Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–88.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74.
Badar T, Burger JA, Wierda WG, O’Brien S. Ibrutinib: a paradigm shift in management of CLL. Expert Rev Hematol. 2014;7:705–17.
Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94.
Anderson JS, Teutsch M, Dong Z, Wortis HH. An essential role for Bruton’s [corrected] tyrosine kinase in the regulation of B-cell apoptosis. Proc Natl Acad Sci U S A. 1996;93:10966–71.
Davids MS, Brown JR. Ibrutinib: a first in class covalent inhibitor of Bruton’s tyrosine kinase. Future Oncol. 2014;10:957–67.
de Weerdt I, Koopmans SM, Kater AP, van Gelder M. Incidence and management of toxicity associated with ibrutinib and idelalisib: a practical approach. Haematologica. 2017;102:1629–39.
Ponader S, Chen SS, Buggy JJ, Balakrishnan K, Gandhi V, Wierda WG, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–9.
Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia. 2014;28:649–57.
Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–37.
Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42.
Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–96.
Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23.
O’Brien S, Jones JA, Coutre SE, Mato AR, Hillmen P, Tam C, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17:1409–18.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16.
Dimopoulos MA, Tedeschi A, Trotman J, García-Sanz R, Macdonald D, Leblond V, et al. Phase 3 trial of ibrutinib plus rituximab in Waldenstrom’s macroglobulinemia. N Engl J Med. 2018;378:2399–410.
Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130:2243–50.
Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–506.
•• Salem JE, Manouchehri A, Bretagne M, et al. Cardiovascular toxicities associated with Ibrutinib. J Am Coll Cardiol. 2019;74:1667–78 Large study evaluating adverse cardiovascular events with ibrutinib and demonstating increaed mortality in the setting of atrial arrhythmais.
Brown JR, Hillmen P, O’Brien S, et al. Extended follow-up and impact of high-risk prognostic factors from the phase 3 RESONATE study in patients with previously treated CLL/SLL. Leukemia. 2018;32:83–91.
Coppens M, Eikelboom JW, Hart RG, Yusuf S, Lip GYH, Dorian P, et al. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34:170–6.
Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2019.
Fradley MG, Gliksman M, Emole J, Viganego F, Rhea I, Welter-Frost A, et al. Rates and risk of atrial arrhythmias in patients treated with ibrutinib compared with cytotoxic chemotherapy. Am J Cardiol. 2019;124:539–44.
Reda G, Fattizzo B, Cassin R, Mattiello V, Tonella T, Giannarelli D, et al. Predictors of atrial fibrillation in ibrutinib-treated CLL patients: a prospective study. J Hematol Oncol. 2018;11:79.
Mato AR, Clasen S, Pickens P, Gashonia L, Rhodes J, Svoboda J, et al. Left atrial abnormality (LAA) as a predictor of ibrutinib-associated atrial fibrillation in patients with chronic lymphocytic leukemia. Cancer Biol Ther. 2018;19:1–2.
Baptiste F, Cautela J, Ancedy Y, Resseguier N, Aurran T, Farnault L, et al. High incidence of atrial fibrillation in patients treated with ibrutinib. Open Heart. 2019;6:e001049.
Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica. 2017;102:1796–805.
McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124:3829–30.
Alomar M, Fradley MG. Electrophysiology translational considerations in cardio-oncology: QT and beyond. J Cardiovasc Transl Res. 2019.
Jiang L, Li L, Ruan Y, Zuo S, Wu X, Zhao Q, et al. Ibrutinib promotes atrial fibrillation by inducing structural remodeling and calcium dysregulation in the atrium. Heart Rhythm. 2019;16:1374–82.
Paydas S. Management of adverse effects/toxicity of ibrutinib. Crit Rev Oncol Hematol. 2019;136:56–63.
Ganatra S, Sharma A, Shah S, Chaudhry GM, Martin DT, Neilan TG, et al. Ibrutinib-associated atrial fibrillation. JACC Clin Electrophysiol. 2018;4:1491–500.
Thompson PA, Levy V, Tam CS, et al. Atrial fibrillation in CLL patients treated with ibrutinib. An international retrospective study. Br J Haematol. 2016;175:462–6.
Rhea IB, Lyon AR, Fradley MG. Anticoagulation of cardiovascular conditions in the cancer patient: review of old and new therapies. Curr Oncol Rep. 2019;21:45.
D’Souza M, Carlson N, Fosbol E, et al. CHA2DS2-VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur J Prev Cardiol. 2018;25:651–8.
Hu WS, Lin CL. Impact of atrial fibrillation on the development of ischemic stroke among cancer patients classified by CHA2DS2-VASc score-a nationwide cohort study. Oncotarget. 2018;9:7623–30.
Vrontikis A, Carey J, Gilreath JA, Halwani A, Stephens DM, Sweetenham JW. Proposed algorithm for managing ibrutinib-related atrial fibrillation. Oncology (Williston Park). 2016;30:970–4 980–1, C3.
Kamel S, Horton L, Ysebaert L, Levade M, Burbury K, Tan S, et al. Ibrutinib inhibits collagen-mediated but not ADP-mediated platelet aggregation. Leukemia. 2015;29:783–7.
Levade M, David E, Garcia C, Laurent PA, Cadot S, Michallet AS, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124:3991–5.
Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–45.
Rhea I, Burgos PH, Fradley MG. Arrhythmogenic anticancer drugs in cardio-oncology. Cardiol Clin. 2019;37:459–68.
Sanz AP, Gomez JLZ. AF in cancer patients: a different need for anticoagulation? Eur Cardiol. 2019;14:65–7.
Lampson BL, Yu L, Glynn RJ, Barrientos JC, Jacobsen ED, Banerji V, et al. Ventricular arrhythmias and sudden death in patients taking ibrutinib. Blood. 2017;129:2581–4.
Tomcsanyi J, Nenyei Z, Matrai Z, Bozsik B. Ibrutinib, an approved tyrosine kinase inhibitor as a potential cause of recurrent polymorphic ventricular tachycardia. JACC Clin Electrophysiol. 2016;2:847–9.
Beyer A, Ganti B, Majkrzak A, Theyyunni N. A perfect storm: tyrosine kinase inhibitor-associated polymorphic ventricular tachycardia. J Emerg Med. 2017;52:e123–7.
•• Guha A, Derbala MH, Zhao Q, et al. Ventricular arrhythmias following Ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol. 2018;72:697–8 Important study quatifying the burden of ventricular arrhythmias associated with ibrutinib exposure.
Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–28.
Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200–11.
Munir T, Brown JR, O’Brien S, Barrientos JC, Barr PM, Reddy NM, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353–63.
O’Brien S, Hillmen P, Coutre S, Barr PM, Fraser G, Tedeschi A, et al. Safety analysis of four randomized controlled studies of ibrutinib in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma or mantle cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18:648–57 e15.
•• Dickerson T, Wiczer T, Waller A et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019. Large study reporting the incidence of hypertension with ibrutinib and its association with adverse cardiovascular outcomes.
Owen C, Berinstein NL, Christofides A, Sehn LH. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr Oncol. 2019;26:e233–40.
Vreman RA, Geenen JW, Hovels AM, Goettsch WG, Leufkens HGM, Al MJ. Phase I/II clinical trial-based early economic evaluation of acalabrutinib for relapsed chronic lymphocytic leukaemia. Appl Health Econ Health Policy. 2019;17:883–93.
Wang M, Rule S, Zinzani PL, Goy A, Casasnovas O, Smith SD, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet. 2018;391:659–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ricardo Pineda-Gayoso declares that he has no conflict of interest.
Mohammed Alomar declares that he has no conflict of interest.
Dae Hyun Lee declares that he has no conflict of interest.
Michael Fradley has received research funding from Medtronic and has received compensation from Novartis for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Pineda-Gayoso, R., Alomar, M., Lee, D.H. et al. Cardiovascular Toxicities of Bruton’s Tyrosine Kinase Inhibitors. Curr. Treat. Options in Oncol. 21, 67 (2020). https://doi.org/10.1007/s11864-020-00764-6
Published:
DOI: https://doi.org/10.1007/s11864-020-00764-6