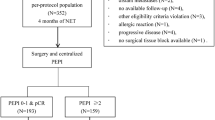
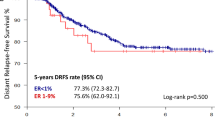
Opinion statement
Neoadjuvant endocrine therapy (NET) with Ki67-based response monitoring is a practical, cost-effective approach to the management of clinical stage II and III estrogen receptor-positive (ER+) breast cancer. In addition to marked improvements in rates of breast conservation, the identification of extreme responders on the basis of the preoperative endocrine prognostic index (PEPI) provides a rationale to avoid chemotherapy on the basis of highly favorable prognosis in some patients. Finally, samples accrued from patients treated with neoadjuvant therapy are providing valuable insights into the molecular basis for intrinsic resistance to endocrine therapy and promise a more rational basis and precise approach to the systemic treatment of ER+ breast cancer.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zardavas D, Irrthum A, Swanton C, Clinical MP. Management of breast cancer heterogeneity. Nat Rev Clin Oncol. 2015;12:381–94.
Kravchenko J, Akushevich I, Seewald V, et al. Breast cancer as heterogeneous disease: contributing factors and carcinogenesis mechanisms. Breast Cancer Res Treat. 2011;128:483–93.
Mauri D, Pavlidis N, Ioannidis P. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Nat Cancer Inst. 2005;97:188–94.
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.
Eiermann W, Pinkowski T, Crown J, et al. Phase III study of doxorubicin/cyclophosphamide with concomitant versus sequential docetaxel as adjuvant treatment in patients with node-positive breast cancer: BCIRG-005 trial. J Clin Oncol. 2011;29:3877–84.
Colleoni M, Viale G, Zahrieh D, Pruneri G, Gentilini O, Veronesi P, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10:6622–8.
Guarneri V, Broglio K, Kau S, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–44.
Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcomes among 100.000 women in 123 randomised trials. Lancet. 2012;379:432–44.
Kwa M, Makris A, Esteva F. Clinical utility of gene-expression signatures in early stage breast cancer. Nat Rev Clin Oncol. 2017;14:595–610.
Smith I. Preoperative endocrine therapy for operable breast cancer. In: Diseases of the breast 5th edition. 2014.
•• Ma C, Reinert T, Chmielewska I, Ellis M. Mechanisms of aromatase inhibitors resistance. Nat Rev Cancer. 2015;15:261–75. Comprehensive review of mechanisms of resistance to AIs cancer considering both genomic and cell biological explanatations as to why ER+ breast cancer cells progress and cause an incurable systemic disease.
Chiba A, Hoskin T, Heins C, Hunt K, Habermann E, Boughey J. Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a national cancer data base study. Ann Surg Oncol. 2017;24:418–24.
Preece P, Wood R, Mackie C, et al. Tamoxifen as initial sole treatment of localised breast cancer in elderly women: a pilot study. Br Med J. 1982;284:869–70.
Gazet J, Markopoulos C, Ford H, Coombes RC, Bland JM, Dixon RC. Prospective randomised trial of tamoxifen versus surgery in elderly patients with breast cancer. Lancet. 1988;1:679–81.
Bates T, RIley D, ea HJ. Breast cancer in elderly women: a cancer research campaign trial comparing treatment with tamoxifen and optimal surgery with tamoxifen alone. Br J Surg. 1991;78:591–4.
Fennessy M, Bates T, MacRae K, Riley D, Houghton J, Baum M, et al. Late follow-up of a randomized trial of surgery plus tamoxifen versus tamoxifen alone in women aged over 70 years with operable breast cancer. Br J Surg. 2004;91:699–704.
Mustacchi G, Ceccherini R, Milani S, Pluchinotta A, de Matteis A, Maiorino L, et al. Tamoxifen alone versus adjuvant tamoxifen for operable breast cancer of the elderly: long-term results of the phase III randomized controlled multicenter GRETA trial. Ann Oncol. 2003;14:414–20.
Fontein D, Chahrehbli A, Nortier W, et al. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients—a phase II trial. Eur J Cancer. 2014;50:2190–200.
Palmieri C, Cleator S, Kliburn L, et al. NEOCENT: a randomised feasibility and translational study comparing neoadjuvant endocrine therapy with chemotherapy in ER-rich postmenopausal primary breast cancer. Breast Cancer Res Treat. 2014;148:581–90.
Alba E, Calvo L, Albanell L, et al. Chemotherapy and hormonetherapy as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase II study. Ann Oncol. 2012;23:3069–74.
Lee B, Liedke P, Barrios C, et al. Breast cancer in Brazil: present status and future goals. Lancet Oncol. 2012;13:e95–102.
Reinert T, Ramalho S, Gonçalves R, Barrios C, Graudenz M, Bines J, et al. Multidisciplinary approach to neoadjuvant endocrine therapy in breast cancer: a comprehensive review. Rev Bras Ginecol Obstet. 2016;38:615–22.
Ellis M, Suman V, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor–rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol. 2011;29:2342–9.
Polley MYLS, Gao D, et al. An international study to increase concordance in Ki67 scoring. Mod Pathol. 2015;10
Reinert T, Barrios C. Optimal management of hormone receptor positive metastatic breast cancer in 2016. Ther Adv Med Oncol. 2015;7:304–20.
(EBCTCG)† EBCTCG. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52.
Smith IE, Dowsett M, Ebbs SR, Dixon JM, Skene A, Blohmer JU, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23:5108–16.
Eiermann W, Paepke S, Appfelstaedt J, Llombart-Cussac A, Eremin J, Vinholes J, et al. Preoperative treatment of postmenopasusal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol. 2001;12:1527–32.
Cataliotti L, Buzdar A, Noguchi S, Bines J, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast Cancer the pre-operative “arimidex” compared to tamoxifen (PROACT) trial. Cancer. 2006;106:2095–103.
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol. 2011;29:2342–9.
Masuda N, Sagara Y, Kinoshita T, Iwata H, Nakamura S, Yanagita Y, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 2012;13:345–52.
Semiglazov V. Neoadjuvant endocrine therapy: exemestane vs tamoxifen in postmenopausal ER+ breast cancer patients (T1–4, N1–2, M0). J Clin Oncol. 2005;23:Abstract 530.
Lerebours F, Bourgier C, Alran S, et al. Abstract PD07-04: a randomized phase II neoadjuvant trial evaluating anastrozole and fulvestrant efficiency for post-menopausal ER-positive, HER2-negative breast cancer patients: first results of the UNICANCER CARMINA 02 French trial. Cancer Res 2012;72(24 Suppl):Abstract nr PD07-04 2012.
Spring L, Gupta A, Reynolds K, et al. Neoadjuvant endocrine therapy for estrogen receptor–positive breast cancer a systematic review and meta-analysis. JAMA Oncol 2016; doi: 101001/jamaoncol20161897 Published online June 30, 2016.
•• Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–8. This paper established a prognostic model for ER+ breast cancer treated with neoadjuvant endocrine therapy based on the biological characteristics and pathological stage of the surgical specimen after completion of preoperative treatment.
DIxon J, Renshhaw L, ea MEJ. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Treat. 2009;113:145–51.
Llombart-Cussac A, Guerrero A, Galan A, et al. Phase II trial with letrozole to maximum response as primary systemic therapy in postmenopausal patients with ER/PgR[+] operable breast cancer. Clin Transl Oncol. 2012;14:125–31.
Krainick-Strobel U, Lichtenegger W, Wallwiener D, et al. Neoadjuvant letrozole in postmenopausal estrogen and/or progesterone receptor positive breast cancer: a phase IIb/III trial to investigate optimal duration of preoperative endocrine therapy. BMC Cancer. 2008;8:62.
Carpenter R, Doughty J, Cordiner C, et al. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Treat. 2014;144:569–76.
Barroso-Sousa R, Silva D, Alessi J, et al. Neoadjuvant endocrine therapy in breast cancer: current role and future perspectives. ecancer. 2016;10:1–15.
• Semiglazov V, Semiglazov V, Dashyan G, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–54. This is one of the few randomized trials that prospectively compared endocrine therapy with chemotherapy in the neoadjuvant setting.
ea COP. LBA9 – Letrozole and palbociclib versus 3rd generation chemotherapy as neoadjuvant treatment of luminal breast cancer. Results of the UNICANCER-NeoPAL study. Ann Oncol. 2017;
Francis P, Regan M, Fleming G, Láng I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–46.
Pagani O, Regan M, Walley B, Fleming GF, Colleoni M, Láng I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–18.
Guidance for Industry. Pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), 2014. at https://www.fda.gov/downloads/drugs/guidances/ucm305501.pdf.)
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32.
Goncalves R, DeSchryver K, Ma C, Tao Y, Hoog J, Cheang M, et al. Development of a Ki-67-based clinical trial assay for neoadjuvant endocrine therapy response monitoring in breast cancer. Breast Cancer Res Treat. 2017;165:355–64.
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J Clin Oncol. 2011;29:2342–9.
•• Ellis MJ, Suman VJ, Hoog J, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol. 2017;35:1061–9. This recently published paper evaluated the role of Ki67 as a surrogate marker for chemotherapy decision during and after NET.
Iwamoto T, Katagiri T, Niikura N, Miyoshi Y, Kochi M, Nogami T, et al. Immunohistochemical Ki67 after short-term hormone therapy identifies low-risk breast cancers as reliably as genomic markers. Oncotarget. 2017;8:26122–8.
Olson JA Jr, Budd GT, Carey LA, et al. Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: results from a multicenter phase II trial. J Am Coll Surg. 2009;208:906–14. discussion 15–6
• Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–70. This publication introduced the role of Ki67 measurement as an early surrogate marker of the pattern of endocrine therapy resistance.
Suman VJ, Ellis MJ, Ma CX. The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol. 2015;4:34.
Cohen AL, Factor RE, Mooney K, Salama ME, Wade M, Serpico V, et al. POWERPIINC (PreOperative window of endocrine therapy provides information to increase compliance) trial: changes in tumor proliferation index and quality of life with 7 days of preoperative tamoxifen. Breast. 2017;31:219–23.
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105:1897–906.
Leung SCY, Nielsen TO, Zabaglo L, et al. Analytical validation of a standardized scoring protocol for Ki67: phase 3 of an international multicenter collaboration. NPJ Breast Cancer. 2016;2:16014.
Polley MY, Leung SC, Gao D, et al. An international study to increase concordance in Ki67 scoring. Mod Pathol. 2015;28:778–86.
• Goncalves R, Reinert T, Ellis MJ. Avoidance of negative results in adjuvant endocrine therapy trials for estrogen receptor-positive breast cancer. J Clin Oncol. 2017;35:2718–9. Short communication about the use of NET studies as a guide to the design of adjuvant endocrine therapy trials in early-stage breast cancer.
Smith IE. Reply to R. Goncalves et al. J Clin Oncol. 2017;35:2719.
Sledge GW Jr. Put some PEPI in your step: Ki67’s long road to respectability. J Clin Oncol. 2017;35:1031–2.
Prat A, Lluch A, Turnbull AK, Dunbier AK, Calvo L, Albanell J, et al. A PAM50-based chemoendocrine score for hormone receptor-positive breast cancer with an intermediate risk of relapse. Clin Cancer Res. 2017;23:3035–44.
Turnbull AK, Arthur LM, Renshaw L, Larionov AA, Kay C, Dunbier AK, et al. Accurate prediction and validation of response to endocrine therapy in breast cancer. J Clin Oncol. 2015;33:2270–8.
Ueno TMN, Yamanaka T, Saji S, Kuroi K, Sato N, et al. Evaluating the 21-gene assay recurrence score® as a predictor of clinical response to 24 weeks of neoadjuvant exemestane in estrogen receptor-positive breast cancer. Int J Clin Oncol. 2014;19:607–13.
Miller CA, Gindin Y, Lu C, Griffith OL, Griffith M, Shen D, et al. Aromatase inhibition remodels the clonal architecture of estrogen-receptor-positive breast cancers. Nat Commun. 2016;7:12498.
Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005.
Goncalves R, Ma C, Luo J, Suman V, Ellis MJ. Use of neoadjuvant data to design adjuvant endocrine therapy trials for breast cancer. Nat Rev Clin Oncol. 2012;9:223–9.
Ma CX, Suman V, Goetz MP, et al. A phase II trial of neoadjuvant MK2206, an AKT inhibitor, with anastrozole in clinical stage 2 or 3 PIK3CA mutant ER positive and HER2 negative breast cancer. Clin Cancer Res. 2017;
Smith IE, Walsh G, Skene A, Llombart A, Mayordomo JI, Detre S, et al. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007;25:3816–22.
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–7.
Baselga J, Campone M, Piccart M, Burris HA III, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9.
Hurvitz SA, Schilder JM, Frenzel M, Martín M. Abstract CT092: a phase II study of neoadjuvant abemaciclib (LY2835219) in postmenopausal women with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) breast cancer (neoMONARCH). Cancer Res. 2016;76:CT092-CT.
•• Ma CX, Gao F, Luo J, et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent Kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23:4055–65. Recently presented trial demonstrating benefit of adding the CDK4/6 inhibitor palbociclib to neoadjuvant AI. The improved efficacy in terms of higher rates of complete cell cycle arrest prompts additional evaluating of this class of drugs in both neoadjuvant and adjuvant trials of early-stage ER+ breast cancer.
Guerrero-Zotano A, Arteaga CL. Neoadjuvant trials in ER+ breast cancer: a tool for acceleration of drug development and discovery. Cancer Discov. 2017;7:1–14.
Polley M-YCLS, McShane LM, Gao D, Hugh JC, Mastropasqua MG, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105:1897–906.
Leung SCYNT, Zabaglo L, Arun I, Badve SS, Bane AL, et al. Analytical validation of a standardized scoring protocol for Ki67: phase 3 of an international multicenter collaboration. npj Breast Cancer. 2016;2:16014.
Focke CMDT, van Diest PJ. Intratumoral heterogeneity of Ki67 expression in early breast cancers exceeds variability between individual tumours. Histopathology. 2016;69:849–61.
Reinert T, Barrios C. Overall survival and progression-free survival with endocrine therapy for hormone receptor-positive, HER2-negative advanced breast cancer: review. Ther Adv Med Oncol. 2017;9:693–709.
Ma CX GF, Luo J, et al: NeoPalAna: neoadjuvant palbociclib, a cyclindependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor positive breast cancer. Clin Cancer Res. 101158/1078-0432CCR-16-3206.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tomás Reinert has received research funding from AstraZeneca and has received speaker’s honoraria from AstraZeneca, Novartis, and Pfizer.
Rodrigo Gonçalves declares that he has no conflict of interest.
Matthew J. Ellis has received clinical trial support from Novartis (P024 and Z1031 trials) and Pfizer (Z1031 trial); has received compensation from AstraZeneca, Pfizer, and Novartis for service as a consultant; and has licensed PAM50 patents to NanoString for Prosigna®. The commercial version of PAM50 is not mentioned within this article.
Human and Animal Rights and Informed Consent
Dr Ellis performed the Preoperative Letrozole Study and the Z1031 Study. He was a coinvestigator of the P024 Study. All these studies on human subjects were approved by the relevant ethics committees as outlined in the publications cited.
Additional information
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Reinert, T., Gonçalves, R. & Ellis, M.J. Current Status of Neoadjuvant Endocrine Therapy in Early Stage Breast Cancer. Curr. Treat. Options in Oncol. 19, 23 (2018). https://doi.org/10.1007/s11864-018-0538-9
Published:
DOI: https://doi.org/10.1007/s11864-018-0538-9