Abstract
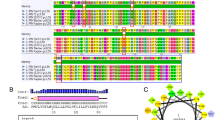
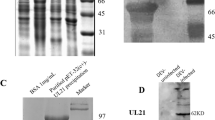
Pseudo rabies virus (PRV) egresses from the nucleus by budding from the inner nuclear membrane (INM). The nuclear lamina forms a rigid meshwork of intermediate filaments underlying the INM. It remains unknown whether PRV infection induces the disruption of lamina. In this paper, it can be observed that nuclear Lamin A became fractured during PRV infection. UL34 was localized at the nuclear rim, but UL31 was accumulated in the nucleus as distinct patches. Interestingly, a part of UL31 was localized at the INM in the presence of UL34. Immunoprecipitation (IP) assay confirmed that PRV UL31 and UL34 interacted in the transfected cells. Importantly, the co-expression of UL31 and UL34 directly disrupted Lamin A, resembling that observed during PRV infection. In conclusion, PRV infection induces the disruption of Lamin A, and UL34 and UL31 play a critical role in the disruption of Lamin A.
Similar content being viewed by others
References
Burke B, Stewart C L. The nuclear lamins: Flexibility in function[J]. Nat Rev Mol Cell Bio, 2013, 14(1): 13–24.
Stuurman N, Maus N, Fisher P A. Interphase phosphorylation of the Drosophila nuclear lamin: Sitemapping using a monoclonal antibody[J]. J Cell Sci, 1995, 108(9): 3137–3144.
Hocevar B A, Burns D J, Fields A P. Identification of protein kinase C (PKC) phosphorylation sites on human lamin B. Potential role of PKC in nuclear lamina structural dynamics[J]. J Biol Chem, 1993, 268(10): 7545–7552.
Reynolds A E, Liang L, Baines J D. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes UL31 and UL34[J]. J Virol, 2004, 78(11): 5564–5575.
Park R, Baines J D. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B[J]. J Virol, 2006, 80(1): 494–504.
Lee C P, Huang Y H, Lin S F, et al. Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production[J]. J Virol, 2008, 82(23): 11913–11926.
Reim N I, Kamil J P, Wang D, et al. Inactivation of retinoblastoma protein does not overcome the requirement for human cytomegalovirus UL97 in lamina disruption and nuclear egress[J]. J Virol, 2013, 87(9): 5019–5027.
Muranyi W, Haas J, Wagner M, et al. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina[J]. Science, 2002, 297(5582): 854–857.
Mettenleiter T C. Aujeszky’s disease (pseudorabies) virus: The virus and molecular pathogenesis——State of the art, June 1999[J], Vet Res, 2000, 31(1): 99–115.
Wu S L, Hsiang C Y, Ho T Y, et al. Identification, expression, and characterization of the pseudorabies virus DNA-binding protein gene and gene product[J]. Virus Res, 1998, 56(1):1–9.
Granzow H, Klupp B G, Fuchs W, et al. Egress of alphaherpesviruses: Comparative ultrastructural study[J]. J Virol, 2001, 75(8): 3675–3684.
Johnson D C, Baines J D. Herpesviruses remodel host membranes for virus egress[J]. Nat Rev Micro, 2011, 9(5): 382–394.
Henaff D, Radtke K, Lippé R. Herpesviruses exploit several host compartments for envelopment[J]. Traffic, 2012, 13(11): 1443–1449.
Klupp B G, Granzow H, Mettenleiter T C. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus[J]. J Gen Virol, 2001, 82(Pt 10): 2363–2371.
Fuchs W, Klupp B G, Granzow H, et al. The UL20 gene product of pseudorabies virus functions in virus egress[J]. J Virol, 1997, 71(7): 5639–5646.
Fuchs W, Granzow H, Klopfleisch R, et al. The UL7 gene of pseudorabies virus encodes a nonessential structural protein which is involved in virion formation and egress[J]. J Virol, 2005, 79(17):11291–11299.
Luxton G W, Lee J I, Haverlock-Moyns S, et al. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport[J]. J Virol, 2006, 80(1): 201–209.
Klupp B G, Granzow H, Mundt E, et al. Pseudorabies virus UL37 gene product is involved in secondary envelopment[J]. J Virol, 2001, 75(19): 8927–8936.
Klupp B G, Baumeister J, Dietz P, et al. Pseudorabies virus glycoprotein gK is a virion structural component involved in virus release but is not required for entry[J]. J Virol, 1998, 72(3): 1949–1958.
Klupp B G, Granzow H, Mettenleiter T C. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product[J]. J Virol, 2000, 74(21): 10063–10073.
Fuchs W, Klupp B G, Granzow H, et al. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not mature virions[J]. J Virol, 2002, 76(1): 364–378.
Paβvogel L, Janke U, Klupp B G, et al. Identification of conserved amino acids in pUL34 which are critical for function of the pseudorabies virus nuclear egress complex[J]. J Virol, 2014, 88(11):6224–6231.
Izumi M, Vaughan O A, Hutchison C J, et al. Head and/or CaaX domain deletions of lamin proteins disrupt preformed Lamin A and C but not Lamin B structure in mammalian cells[J]. Mol Biol Cell, 2000, 11(12):4323–4337.
Camozzi D, Pignatelli S, Valvo C, et al. Remodelling of the nuclear lamina during human cytomegalovirus infection: Role of the viral proteins pUL50 and pUL53[J]. J Gen Virol, 2008, 89(3):731–740.
Scott ES, O’Hare P. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection[J]. J Virol, 2001, 75(18): 8818–8830.
Paβvogel L, Trübe P, Schuster F, et al. Mapping of sequences in pseudorabies virus pUL34 that are required for formation and function of the nuclear egress complex[J]. J Virol, 2013, 87(8): 4475–4485.
Van Minnebruggen G, Favoreel H W, Jacobs L, et al. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown[J]. J Virol, 2003, 77(16): 9074–9080.
Jacob T, Van den Broeke C, Grauwet K, et al. Pseudorabies virus US3 leads to filamentous actin disassembly and contributes to viral genome delivery to the nucleus[J]. Vet Microbiol, 2015, 177 (3-4): 379–385.
Favoreel H W, Van Minnebruggen G, Adriaensen D, et al. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread[J]. Proc Natl Acad Sci USA, 2005, 102 (25): 8990–8995.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Supported by the National Natural Science Foundation of China (31501701, 31371386), the Plant Foundation for Young Scientists of Henan University (CX0000A40557)
Rights and permissions
About this article
Cite this article
Wei, W., Hu, Z., Kang, X. et al. Pseudo Rabies Virus Protein UL34 Interacted with UL31 to Disrupt the Human Lamin A. Wuhan Univ. J. Nat. Sci. 23, 454–460 (2018). https://doi.org/10.1007/s11859-018-1347-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11859-018-1347-5