Abstract
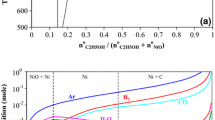
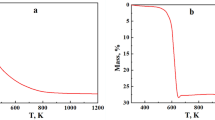
The reduction behavior of SnO2 powder has been investigated in the temperature range of 900–1200 K under ethanol flow. Scanning electron microscopy, x-ray diffraction, and mass measurement techniques were used to characterize the products. Full oxide reduction was attained at 1000 K, 1100 K, and 1200 K within about 20 min, 15 min, and 7.5 min, respectively. At 900 K, the extent of reduction increased with the reaction time up to 20 min, but further increases in the time (30 min and 60 min) resulted in a slight mass gain. This was attributable to the C uptake. Spherical Sn particles (diameter ~ 1 μm) were observed at 1000 K and 1100 K. At 1200 K, large beads of Sn (diameter 400–800 μm) were obtained. The spherical particle morphology was attributed to the liquid metallic phase formed during the reaction. The reduction mechanism of SnO2 in ethanol has been discussed in the light of thermodynamic and experimental results.




Similar content being viewed by others
References
W.B. Hampshire, ASM Handbook, Volume 2—Properties and Selection: Nonferrous Alloys and Special-Purpose Materials, ed. J.R. Davis (Materials Park, OH: ASM International, 1990), p. 517.
B.S. Kim, J.C. Lee, H.S. Yoon, and S.K. Kim, Mater. Trans. 52, 1814 (2011).
S. Cetinkaya and S. Eroglu, Int. J. Miner. Process. 110–111, 71 (2012).
H. Ha, M. Yoo, H. An, K. Shin, T. Han, Y. Sohn, S. Kim, S.R. Lee, J.H. Han, and H.Y. Kim, Sci. Rep. 7, 1 (2017).
F. Korkmaz, S. Cetinkaya, and S. Eroglu, Metall. Mater. Trans. B 47B, 2378 (2016).
F. Coskun, S. Cetinkaya, and S. Eroglu, JOM 69, 987 (2017).
S. Cetinkaya and S. Eroglu, Int. J. Refract. Metall. Hard Mater. 64, 184 (2017).
G. Eriksson, Chem. Scr. 8, 100 (1975).
M.C. Altay and S. Eroglu, Metall. Mater. Trans. B 48B, 2067 (2017).
S. Cetinkaya and S. Eroglu, J. Eur. Ceram. Soc. 31, 869 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cetinkaya, S., Eroglu, S. Synthesis of Tin Powder Using Tin Oxide and Ethanol. JOM 70, 656–660 (2018). https://doi.org/10.1007/s11837-018-2781-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-018-2781-8