Abstract
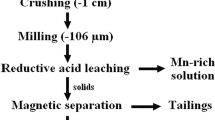
With the depletion of high-grade manganese ores, Mn ore tailings are considered valuable secondary resources. In this study, a process combining high-gradient magnetic separation (HGMS) with hydrometallurgical methods is proposed to recycle fine-grained Mn tailings. The Mn tailings were treated by HGMS at 12,500 G to obtain a Mn concentrate of 30% Mn with the recovery efficiency of 64%. The Mn concentrate could be used in the ferromanganese industry. To recover Mn further, the nonmagnetic fraction was leached by SO2 in an H2SO4 solution. Hydrogen peroxide was added to the leachate to oxidize Fe2+ to Fe3+, and the solution pH was adjusted to 5.0–5.5 with ammonia to remove Al, Fe, and Si impurities. The purified solution was reacted with NH4HCO3, and a saleable product of MnCO3 with 97.9% purity was obtained. The combined process can be applied to Mn recovery from finely dispersed weakly magnetic Mn ores or tailings.



Similar content being viewed by others
References
K. Hagelstein, J. Environ. Manag. 90, 3736 (2009).
W. Zhang and C.Y. Cheng, Hydrometallurgy 89, 137 (2007).
Y. Wu, B. Shi, W. Ge, C.J. Yan, and X. Yang, JOM 67, 361 (2015).
S.K. Tripathy, P.K. Banerjee, and N. Suresh, Int. J. Miner. Metall. Mater. 22, 661 (2015).
G.V. Rao, B.K. Mohapatra, and A.K. Tripathy, Magn. Electron. Sep. 9, 69 (1998).
M. Mpho, B. Samson, and A. Ayo, Int. J. Min. Sci. Technol. 23, 537 (2013).
Z.L. Cai, Y.L. Feng, H.R. Li, X.W. Liu, and Z.C. Yang, JOM 64, 1296 (2012).
W.Y. Sun, S.J. Su, Q.Y. Wang, and S.L. Ding, Hydrometallurgy 133, 118 (2013).
S.C. Das, P.K. Sahoo, and P.K. Rao, Hydrometallurgy 8, 35 (1982).
A.A. Nayl, I.M. Ismail, and H.F. Aly, Int. J. Miner. Process. 100, 116 (2011).
D. Hariprasad, B. Dash, M.K. Ghosh, and S. Anand, Miner. Eng. 20, 1293 (2007).
A. Alaoui, K.E. Kacemi, K.E. Ass, S. Kitane, and S.E. Bouzidi, JOM 67, 1086 (2015).
F. Vegliò, M. Trifoni, F. Pagnanelli, and L. Toro, Hydrometallurgy 60, 167 (2001).
B. Xin, T. Li, X. Li, Z. Dan, F. Xu, N. Duan, Y. Zhang, and H. Zhang, J. Clean. Prod. 92, 54 (2015).
S. Ghosh, S. Mohanty, A. Akcil, L.B. Silcla, and A.P. Das, Chemosphere 154, 628 (2016).
J. Svoboda, Phys. Sep. Sci. Eng. 2, 51 (1986).
Y. Zeng and T. Liu, Ore Geol. Rev. 15, 153 (1999).
S. Pani, S.K. Singh, and B.K. Mohapatra, JOM 68, 1 (2016).
Acknowledgements
This work is financially supported by the Special Fund for Research in the Public Interest of Ministry of Land and Resources of China (Grant No. 201211069).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Tan, X., Yi, Y. et al. Recovery of Manganese Ore Tailings by High-Gradient Magnetic Separation and Hydrometallurgical Method. JOM 69, 2352–2357 (2017). https://doi.org/10.1007/s11837-017-2521-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-017-2521-5