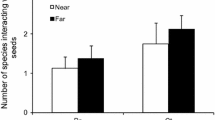
Abstract
The direct and indirect interactions of invasive ants with plants, insect herbivores, and Hemiptera are complex. While ant and Hemiptera interactions with native plants have been well studied, the effects of invasive ant–scale insect mutualisms on the reproductive output of invasive weeds have not. The study system consisted of Argentine ants (Linepithema humile), boneseed (Chrysanthemoides monilifera monilifera), and sap-sucking scale insects (Hemiptera: Saissetia oleae and Parasaissetia nigra), all of which are invasive in New Zealand. We examined the direct and indirect effects of Argentine ants on scale insects and other invertebrates (especially herbivores) and on plant reproductive output. Argentine ants spent one-third of their time specifically associated with scale insects in tending behaviours. The invertebrate community was significantly different between uninfested and infested plants, with fewer predators and herbivores on ant-infested plants. Herbivore damage was significantly reduced on plants with Argentine ants, but sooty mould colonisation was greater where ants were present. Herbivore damage increased when ants were excluded from plants. Boneseed plants infested with Argentine ants produced significantly more fruits than plants without ants. The increase in reproductive output in the presence of ants may be due to increased pollination as the result of pollinators being forced to relocate frequently to avoid attack by ants, resulting in an increase in pollen transfer and higher fruit/seed set. The consequences of Argentine ant invasion can be varied; not only does their invasion have consequences for maintaining biodiversity, ant invasion may also affect weed and pest management strategies.

Similar content being viewed by others
References
Abbott KL, Green PT (2007) Collapse of an ant–scale mutualism in a rainforest on Christmas Island. Oikos 116:1238–1246
Altshuler DL (1999) Novel interactions of non-pollinating ants with pollinators and fruit consumers in a tropical forest. Oecologia 119:600–606
Bach CE (1991) Direct and indirect interactions between ants (Pheidole megacephala), scales (Coccus viridis) and plants (Pluchea indica). Oecologia 87:233–239
Beggs JR (2001) The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abundant carbohydrate resource. Biol Conserv 99:17–28
Blancafort X, Gomez C (2005) Consequences of the Argentine ant, Linepithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae). Acta Oecol 28:49–55
Briden K (2008) Current status and management of boneseed in New Zealand. Plant Prot Q 23(1):20–22
Buckley RC (1987) Interactions involving plants, Homoptera, and ants. Annu Rev Ecol Syst 18:111–135
Chamberlain SA, Holland JN (2009) Quantitative synthesis of context-dependency in ant-plant protection mutualisms. Ecology 90(9):2384–2392
Clarke KR, Warwick RM (2005) Change in marine communities. An approach to statistical analysis and interpretation, 2nd edn. Plymouth Marine Laboratory, Plymouth
Denno RF, McClure MS, Ott JR (1995) Interspecific interactions in phytophagous insects: competition revisited and resurrected. Ann Rev Entomol 40:297–331
Gastreich KR (1999) Trait-mediated indirect effects of a Theridiid spider on an ant–plant mutualism. Ecology 80:1066–1070
Green OR (1990) Entomologist sets new record at Mt Smart for Iridomyrmex humilis established in New Zealand. Weta 13:14–16
Harris R, Ward D, Sutherland MA (2002) A survey of the current distribution of Argentine ants, Linepithema humile, in native habitats in New Zealand, and assessment of future risk of establishment. Landcare Research Contract Report LC0102/105 to Ministry of Agriculture and Forestry, Biosecurity Authority. http://argentineants.landcareresearch.co.nz/documents/Harris_etal_2002_MAF_report.pdf Accessed 20/3/12
Helms KR, Vinson SB (2003) Apparent facilitation of an invasive mealybug by an invasive ant. Insectes Soc 50:403–404
Hodgson CJ, Henderson RC (2000) Coccidae (Insecta: Hemiptera: Coccoidea). Fauna of New Zealand 41. Manaaki Whenua Press, Lincoln
Hoeksema JD, Bruna EM (2000) Pursuing the big questions about interspecific mutualism: a review of theoretical approaches. Oecologia 125:321–330
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, New Jersey
Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328
Krivan V, Schmitz OJ (2004) Trait and density mediated indirect interactions in simple food webs. Oikos 107:239–250
Lach L (2003) Invasive ants: unwanted partners in ant–plant interactions. Ann Mo Bot Gard 90:95–108
Lach L (2007) A mutualism with a native membracid facilitates pollinator displacement by Argentine ants. Ecology 88:1994–2004
Lach L (2008) Argentine ants displace floral arthropods in a biodiversity hotspot. Divers Distrib 14:281–290
Lach L, Tillberg CV, Suarez AV (2010) Contrasting effects of an invasive ant on a native and an invasive plant. Biol Invasions 12:3123–3133
Meyer GA (2000) Effects of insect feeding on growth and fitness of goldenrod (Solidago altissima). Recent Res Develop Entomol 3:29–41
Ness JH (2006) A mutualism’s indirect costs: the most aggressive plant bodyguards also deter pollinators. Oikos 113:506–514
Ness JH, Bronstein JL (2004) The effects of invasive ants on prospective ant mutualists. Biol Invas 6:445–461
Norbury G (2001) Conserving dryland lizards by reducing predator-mediated apparent competition and direct competition with introduced rabbits. J Appl Ecol 38:1350–1361
O’Dowd DJ, Green PT, Lake PS (2003) Invasional ‘meltdown’ on an oceanic island. Ecol Lett 6:812–817
Rooney TP, Waller DM (2003) Direct and indirect effects of white-tailed deer in forest ecosystems. For Ecol Manage 181:165–176
Schoener TW (1993) On the relative importance of direct versus indirect effects in ecological communities. In: Kawanabe H, Cohen JE, Iwasaki K (eds) Mutualism and community organization: behavioral, theoretical, and food web approaches. Oxford University Press, Oxford, pp 365–411
Schoener TW, Spiller DA, Losos JB (2002) Predation on a common Anolis lizard: can the food-web effects of a devastating predator be reversed? Ecol Monogr 72:383–407
Siddon CE, Witman JD (2004) Behavioral indirect interactions: multiple predator effects and prey switching in shallow rocky subtidal zone. Ecology 85:2938–2945
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invas 1:21–32
Stanley MC, Ward DF (2012) Impacts of Argentine ants on invertebrate communities with below-ground consequences. Biodivers Conserv (in press)
Stanley MC, Ward DF, Harris RJ, Arnold G, Toft R, Rees J (2008) Optimising pitfall sampling for the detection of Argentine ants, Linepithema humile (Hymenoptera: Formicidae). Sociobiol 51:461–472
Strauss SY (1991) Indirect effects in community ecology: their definition, study and importance. Trends Ecol Evol 6:206–210
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Styrsky JD, Eubanks MD (2007) The ecological consequences of ant-hemipteran interactions. Proc Roy Soc B 274:151–164
Styrsky JD, Eubanks MD (2010) A facultative mutualism between aphids and an invasive ant increases plant reproduction. Ecol Entomol 35:190–199
Trager MD, Bhotika S, Hostetler JA, Andrade GV, Rodriguez-Cabal MA, McKeon CS, Osenberg CW, Bolker BM (2010) Benefits for plants in ant-plant protective mutualisms: a meta-analysis. PLoS One 5(12):e14308. doi:10.1371/journal.pone.0014308
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Tree 21:208–216
Veech JA (2000) Predator-mediated interactions among the seeds of desert plants. Oecologia 124:402–407
Ward DF, Harris R (2005) Invasibility of native habitats by Argentine ants, Linepithema humile, in New Zealand. NZ J Ecol 29:215–219
Ward DF, Harris RJ, Stanley MC (2005) Human-mediated range expansion of Argentine ants Linepithema humile (Hymenoptera: Formicidae) in New Zealand. Sociobiology 45:401–408
Ward DF, Green C, Harris RJ, Hartley S, Lester PJ, Stanley MC, Suckling DM, Toft RJ (2010) Twenty years of Argentine ants in New Zealand: past research and future priorities for applied management. NZ Entomol 33:67–78
Way MJ (1963) Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol 8:307–344
Weiss PW, Adair PB, Edwards MA, Winkler MA, Downey PO (2008) Chrysanthemoides monilifera subsp. monilifera (l.) T. Norl. and subsp. rotundata (DC.) T. Norl Plant Prot Q 23:3–14
Winks CJ, Fowler SV, Smith LA (2004) Invertebrate fauna of boneseed, Chrysanthemoides monilifera ssp. monilifera (L.) T. Norl. (Asteraceae: Calenduleae), an invasive weed in New Zealand. NZ Entomol 27:61–72
Wootton JT (1994) The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Syst 25:443–466
Acknowledgments
We thank the Auckland City Council for permission to work in Te Whau Point reserve. We also thank Jo Rees for data checking, Rosa Henderson for identifying the scale insects, and Stephen Thorpe for identifying other invertebrates. Greg Arnold and Guy Forrester helped with statistical design and analyses. Richard Toft, Shaun Forgie and two anonymous reviewers improved earlier versions of the manuscript. This work was funded by the University of Auckland summer student scholarship programme (to L. Phillips, H. Nathan, J. Galbraith, S. Knight) and the New Zealand Foundation for Research, Science and Technology (C09X0211) and complies with New Zealand laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Kris Wyckhuys.
Rights and permissions
About this article
Cite this article
Stanley, M.C., Nathan, H.W., Phillips, L.K. et al. Invasive interactions: can Argentine ants indirectly increase the reproductive output of a weed?. Arthropod-Plant Interactions 7, 59–67 (2013). https://doi.org/10.1007/s11829-012-9215-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-012-9215-2