Abstract
Objective
The objective of this study was to test the hypothesis that ST elevation myocardial infarction (STEMI) patients with sleep-disordered breathing (SDB) have less resolution of ST segment deviation and more microvascular obstruction (MVO) after percutaneous coronary intervention (PCI) than STEMI patients without SDB.
Methods
In this prospective observational study, patients with STEMI and successful PCI were stratified based on the presence (apnea–hypopnea index [AHI] ≥ 15/h) or absence (AHI < 15/h) of SDB. ST deviations in electrocardiograms (ECGs) were analyzed according to standard criteria before PCI, and at 0–24 h and >24 h after PCI. MVO was assessed by cardiac magnetic resonance imaging.
Results
A total of 35 patients were enrolled, 18 with SDB and 17 with no SDB. Before PCI, median ST deviation was similar in the no SDB and SDB groups (0.094 [0.063–0.144] vs. 0.106 [0.055–0.132] mV, p = 0.88). The no SDB group had significantly less residual ST deviation compared to the SDB group, both within 24 h (0.018 [0.012–0.039] vs. 0.052 [0.035–0.077] mV, p = 0.01) and more than 24 h after PCI (0.016 [0.005–0.029] vs. 0.045 [0.017–0.097] mV, p = 0.006). Multivariable linear regression models including established determinants for infarct size and myocardial ischemia showed that AHI was independently associated with higher ST deviation >24 h after STEMI (B [95% confidence interval, CI] 0.82 [−0.12; 1.51], p = 0.024) and MVO (B [95% CI] 0.08 [0.01; 0.160]; p = 0.036).
Conclusion
SDB is associated with a lower relative reduction in ST deviations and more MVO after STEMI. The present findings suggest that SDB-related myocardial ischemia on the myocardium at risk takes place in the first days after STEMI.
Zusammenfassung
Ziel
Ziel der vorliegenden Studie war es, die Hypothese zu untersuchen, dass Patienten mit ST-Hebungs-Infarkt („ST elevation myocardial infarction“, STEMI) und schlafbezogener Atmungsstörung (SBA) seltener eine Rückbildung der ST-Strecken-Veränderung und häufiger eine mikrovaskuläre Obstruktion (MVO) nach perkutaner Koronarintervention (PCI) aufweisen als STEMI-Patienten ohne SBA.
Methoden
In der vorliegenden prospektiven Beobachtungsstudie wurden Patienten mit STEMI und erfolgreicher PCI – je nach Vorliegen einer SBA (Apnoe-Hypopnoe-Index, AHI: ≥ 15/h) oder nicht (AHI < 15/h) – stratifiziert. Die ST-Abweichungen im Elektrokardiogramm (EKG) wurden entsprechend den Standardkriterien vor PCI sowie 0–24 h und >24 h nach PCI ausgewertet. Die Beurteilung einer MVO erfolgte anhand der kardialen Magnetresonanztomographie.
Ergebnisse
Es wurden 35 Patienten in die Studie aufgenommen, 18 mit SBA und 17 ohne SBA. Vor der PCI war die mediane ST-Abweichung in der Gruppe ohne SBA ähnlich wie in der Gruppe mit SBA (0,094 [0,063–0,144] vs. 0,106 [0,055–0,132] mV; p = 0,88). Die Gruppe ohne SBA wies signifikant weniger residuale ST-Abweichungen auf als die Gruppe mit SBA, sowohl innerhalb von 24 h (0,018 [0,012–0,039] vs. 0,052 [0,035–0,077] mV; p = 0,01) als auch mehr als 24 h nach PCI (0,016 [0,005–0,029] vs. 0,045 [0,017–0,097] mV, p = 0,006). Multivariate lineare Regressionsmodelle einschließlich etablierter Determinanten für die Infarktgröße und Myokardischämie zeigten, dass der AHI in unabhängiger Weise mit einer größeren ST-Abweichung >24 h nach STEMI (B [95 %-Konfidenzintervall, 95 %-KI] 0,82 [−0,12; 1,51]; p = 0,024) und einer MVO (B [95 %-KI] 0,08 [0,01; 0,160]; p = 0,036) assoziiert war.
Schlussfolgerung
SBA sind mit einer geringeren relativen Rückbildung der ST-Abweichungen und mit einer größeren MVO nach STEMI assoziiert. Die vorliegenden Befunde weisen darauf hin, dass eine SBA-bedingte Myokardischämie im entsprechend gefährdeten Myokard in den ersten Tagen nach STEMI auftritt.
Similar content being viewed by others
Statement of significance
What is already known about this subject?
After ST elevation myocardial infarction treated with percutaneous coronary intervention, sleep-disordered breathing is associated with repetitive hypoxia, increased cardiac workload, and less myocardial salvage.
What does this study add?
Patients with sleep-disordered breathing have prolonged resolution of ST deviation and higher frequencies of microvascular obstruction in the first days after ST elevation myocardial infarction.
How might this impact on clinical practice?
In an ongoing multicenter randomized controlled trial of adaptive servoventilation in patients with acute myocardial infarction and sleep-disordered breathing (TEAM-ASV; NCT02093377) the question is investigated whether prompt diagnosis and early treatment can prevent further myocardial damage after acute myocardial infarction.
Introduction
In patients with ST elevation myocardial infarction (STEMI), prognosis is highly dependent on the extent of the necrotic area in the myocardium [1]. Timely reperfusion of the infarcted area using percutaneous coronary intervention (PCI) is the clinical standard to limit expansion of infarct size [2]. Salvage of vital myocardium and limitation of the myocardial necrotic area contributes to improved prognosis [2, 3]. After successful PCI resulting in reperfusion of the infarcted myocardium, necrotic areas and vital myocardium at risk for necrosis can coexist [4].
An early and well-known marker of the extent of myocardial ischemia in patients with STEMI is ST segment deviation on an electrocardiogram (ECG) [5, 6]. The extent and early resolution of ST segment deviation, as well as residual ST deviation, are strong predictors of prognosis in patients with STEMI [7, 8] and correlate with infarct size and microvascular obstruction (MVO) assessed by cardiovascular magnetic resonance (CMR) [9, 10].
Sleep-disordered breathing (SDB) is common in patients with acute myocardial infarction, with a prevalence of up to 66% [11,12,13]. It is known that SDB is associated with nocturnal myocardial ischemia [14] and is an independent predictor for the occurrence of acute myocardial infarction [15, 16]. SDB results in repetitive oxygen desaturations, as well as surges in heart rate and blood pressure and thus in cardiac workload [17]. The resulting mismatch of oxygen supply and demand associated with SDB may have additional negative effects on myocardium at risk that could be possibly salvaged [17]. Thus, SDB is associated with impaired recovery of left and right ventricular function [18, 19], myocardial salvage, and larger infarct size after acute myocardial infarction [20], as determined by CMR. Untreated pre-existing SDB is also associated with less resolution of ST elevation in STEMI patients assessed 30 min after PCI [21]. However, it is not known whether exposure to SDB harms the myocardium at risk in STEMI patients by promoting continued ischemia within the first days after successful PCI and whether SDB is associated with MVO.
Thus, the objective of this study was to test the hypothesis that STEMI patients with SDB have a higher extent of residual ST segment deviation after PCI and more MVO than similar patients without SDB.
Methods
Study design
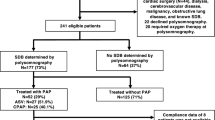
This analysis was undertaken as part of a prospective observational study from March 2009 to December 2011 in patients with first acute myocardial infarction (ST elevation on ECG, or complete occlusion of coronary artery in non-STEMI) who were admitted to the Universitätsklinikum Regensburg and underwent a primary PCI within the first 24 h after symptom onset [20] (Supplementary Figure S1). Key exclusion criteria were a previous myocardial infarction or previous myocardial revascularization, indication for surgical myocardial revascularization, cardiogenic shock, contraindications for CMR, known and treated SDB, and follow-up not feasible [20]. For this subanalysis, patients with non-STEMI were excluded.
Within the first 5 days after PCI, all eligible patients underwent an overnight in-laboratory sleep study (polysomnography). CMR studies were performed on days 3–5 after PCI and 12 weeks later [20]. Data from routine ECGs performed as part of regular clinical practice immediately before PCI until 5 days after PCI were used for the present analysis.
The primary endpoint of this analysis was the extent of residual ST deviation within 24 h and more than 24 h after PCI. This primary endpoint was chosen because high residual ST deviation indicates prolonged ischemia and is associated with worse prognosis [22]. Secondary endpoints were the relative resolution of ST deviation, the extent of MVO, and the N‑terminal pro-brain natriuretic peptide (NT-proBNP) concentration before and after PCI.
Percutaneous coronary intervention and medical management of STEMI
PCI and medical management of STEMI were performed according to contemporary guidelines [23]. The use of thrombectomy and glycoprotein IIb/IIIa inhibitors was at the discretion of the interventional cardiologist, depending on the presence of high thrombus burden in the infarct-related artery. All patients were treated with aspirin, intravenous heparin, and adenosine diphosphate (ADP) receptor inhibitors.
Polysomnography
Polysomnography was performed in all subjects using standard techniques (Alice System, Respironics, Pittsburgh, USA) [20]. Respiratory efforts were measured with the use of respiratory inductance plethysmography, and airflow by nasal pressure cannula. Sleep stages, arousals, and apneas and hypopneas were determined according to American Academy of Sleep Medicine (AASM) criteria by one experienced sleep technician blinded to the clinical data [24]. Apnea was defined as a cessation of inspiratory airflow for ≥10 s. A hypopnea required a 30% or more reduction in nasal pressure signal associated with a ≥4% desaturation (hypopnea definition A, AASM 2007) [24]. The apnea–hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour of sleep. An AHI of ≥15 events per hour indicates at least a moderate degree of SDB. Patients were stratified into those without (AHI < 15/h) and with SDB (AHI ≥ 15/h) [25].
ECG acquisition and analysis
ST deviations in routine 12-lead ECGs were analyzed according to standard criteria [8, 26] before PCI, at 0–24 h, and more than 24 h after PCI. Elevations and depressions of the ST segment were measured according to infarct location in all affected leads of the ECG. The analysis of ST deviations was blinded to other clinical data.
The extent of ST deviation was measured at the J point. Mean ST deviation was calculated according to infarct location. In order to obtain the most accurate results, ST depression in reciprocal leads was also taken into consideration [8]. For anterior infarction, mean ST deviation was calculated as the mean of ST elevations in leads I, aVL, V1–V6, and reciprocal ST depressions in the leads II, III, and aVF. For non-anterior infarctions, mean ST deviation was calculated as the mean of ST elevations in leads II, III, aVF, V5, and V6, and reciprocal ST depressions in leads V1–V4 [8]. Routine clinical ECGs were grouped into three time periods. Relative resolution of ST deviation was categorized into three groups of prognostic importance: complete resolution, partial resolution, and no resolution [7, 8]. Complete resolution was defined as ≥70% resolution of ST deviation compared to ECG before PCI, partial resolution as >30% to <70% resolution of ST deviation, and no resolution as <30% resolution of ST deviation [7].
To measure the extent of ST deviation in digitalized 12-lead ECGs, a program was used which performs as a digital magnifier and enables precise measurement of ST deviation with an accuracy of 0.001 mV (Measure 2.1 d, DatInf GmbH, Tübingen, Germany).
Cardiovascular magnetic resonance acquisition and analysis
Details of CMR acquisition and analysis have been published previously [20]. Briefly, CMR studies were performed on a clinical 1.5-Tesla scanner (Avanto, Siemens Healthcare Sector, Erlangen, Germany) using a 32-channel phased-array receiver coil. Calculation of left ventricular (LV) volumes was performed in serial short-axis slices using commercially available software (Syngo Argus, version B15; Siemens Healthcare Sector, Erlangen, Germany). The extent of delayed enhancement was quantified with custom analysis software (VPT, Siemens Corporate Research, Princeton, NJ, USA) [27]. On the delayed enhancement imaging, myocardial infarction was defined as a signal intensity of hyperenhanced myocardium greater than five standard deviations (SD) above the mean signal intensity of the remote region [20], and MVO was defined as a hypoenhanced region within infarcted myocardium. All measurements were expressed as a percentage of the total LV myocardial volume.
Statistical analysis
Normally distributed quantitative data are expressed as mean ± SD. Non-normally distributed quantitative data are expressed as median and interquartile range. Categorical data are expressed as frequencies with percentages. Comparison of quantitative variables between SDB and no SDB patients were made using an unpaired Student’s t-test for normally distributed variables or a non-parametric statistical test (Mann–Whitney) for skewed variables. Comparison between categorical variables was performed using the exact unconditional Pearson chi-squared statistic.
Multivariable linear regression analyses were performed to identify predictors of ST deviation within 24 h and after more than 24 h post PCI, and for MVO in baseline CMR. Known potential confounders and risk factors that can affect myocardial ischemia [28, 29] and infarct size [20, 30] were entered into the adjusted models. Categorical variables included infarct location, TIMI flow before and after PCI, and current smoking. Continuous variables included symptom onset to reperfusion time, ST deviation before PCI, and AHI/h.
For graphical illustration, boxplots and scatterplots were used. All reported p-values were two-sided, and a p-value of 0.05 was considered the threshold for statistical significance. Data entry and calculation were performed with the software package SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Results
In total, 57 STEMI patients fulfilled the inclusion and exclusion criteria of the observational study [20], of whom 35 could be included in the current analysis (Supplementary Figure S1). Of these patients, 18 had an AHI ≥15/h and were included in the SDB group. ST deviation pre PCI was measured in the ECG which was performed immediately before PCI. Mean time of measurement was similar in the no SDB and the SDB groups for the periods 0–24 h post PCI (5.1 ± 5.5 vs. 5.2 ± 4.2 h, p = 0.964) and >24 h post PCI (87.3 ± 48.3 vs. 88.2 ± 36.8 h, p = 0.953).
Patient characteristics
Baseline patient, sleep, and respiratory characteristics are presented in Table 1. There were no significant differences between patients with and without SDB with respect to age, gender, body mass index, coronary risk factors, and hemodynamic findings. There were also no significant differences between groups for time from symptom onset to revascularization, infarct-related artery, TIMI flow pre or post PCI, thrombus aspiration, or use of glycoprotein IIb/IIIa inhibitors during PCI. All patients were receiving similar medical therapy.
ST deviation and relative resolution of ST deviation
The extent of ST deviation before PCI was similar in the no SDB and SDB groups (0.094 [0.063–0.144] vs. 0.106 [0.055–0.132] mV, p = 0.88; Fig. 1). The no SDB group had significantly lower ST deviation compared to the SDB group both within the first 24 h after PCI (0.018 [0.012–0.039] vs. 0.052 [0.035–0.077] mV, p = 0.01) and more than 24 h after PCI (0.016 [0.005–0.029] vs. 0.045 [0.017–0.097] mV, p = 0.006).
Relative resolution of ST deviation was significantly higher in the no SDB group than in the SDB group within the first 24 h after PCI (74 [58–89] vs. 51 [24–69]%, p = 0.004; Fig. 2) and more than 24 h post PCI (86 [67–95] vs. 50 [13–84]%, p = 0.037). Complete resolution of ST deviation occurred significantly more frequently in the no SDB group than in the SDB group (0–24 h post PCI: 65 vs. 22%, p = 0.011; >24 h post PCI: 76 vs. 39%, p = 0.025; Supplementary Figure S2).
Although ST deviation pre PCI and 0–24 h post PCI did not correlate with the final infarct size determined by CMR 12 weeks after infarction, there was a significant correlation between 12-week infarct size and ST deviation at more than 24 h after PCI (r = 0.751, p < 0.001; Fig. 3).
Microvascular obstruction
After PCI the no SDB group showed significantly lower MVO compared to the SDB group (0.0 [0.0–1.07] vs. 1.74 [0.23–6.16]%, p = 0.027; Fig. 4). The extent of MVO was significantly correlated with the extent of ST deviation at more than 24 h post PCI (r = 0.496, p = 0.005). There was also a significant correlation between MVO at baseline and the infarct size at 12 weeks (r = 0.754, p < 0.001).
NT-proBNP
There was no significant difference between the no SDB and SDB groups in NT-proBNP concentrations before PCI (108 [38–995] vs. 195 [51–1150] pg/mL, p = 0.64) and within the first 24 h after PCI (765 [463–1351] vs. 1328 [777–3142] pg/mL, p = 0.19; Fig. 5). However, at more than 24 h after PCI, the no SDB group had a significantly lower NT-proBNP concentration (810 [326–1000] vs. 1231 [690–2229] pg/mL, p = 0.026).
Predictors of ST deviation and MVO
Multiple linear regression analyses were used to identify independent predictors of residual ST deviation within 24 h and more than 24 h after PCI and MVO (Table 2). Symptom-to-balloon time and ST deviation pre PCI were independent predictors of ST deviation 0–24 h after PCI. Symptom-to-balloon time was also an independent predictor of ST deviation more than 24 h after PCI, whereas ST deviation pre PCI was not an independent predictor of ST deviation more than 24 h after PCI. Other independent predictors for ST deviation more than 24 h after PCI were infarct location, TIMI flow pre and post PCI, and current smoking. Although AHI was not an independent predictor of ST deviation 0–24 h post PCI, there was a significant association between AHI and ST deviation more than 24 h after PCI. Significant predictors of MVO were TIMI flow post PCI and AHI.
Discussion
This study reports a number of major novel observations. First, patients with SDB had a higher absolute ST deviation and less relative resolution of ST deviation compared to patients without SDB within the first 24 h and more than 24 h after PCI; this finding was independent of known risk factors for myocardial ischemia and infarct size. Secondly, residual ST deviation more than 24 h after PCI was significantly associated with final infarct size after 12 weeks. Thirdly, early after PCI, MVO was more frequent and affected more myocardium in patients with SDB than in those without SDB; the number of apneas and hypopneas per hour (AHI) was an independent predictor of MVO early after PCI. Finally, patients with SDB had higher serum levels of NT-proBNP more than 24 h after PCI.
A previous study by Nakashima et al. showed that STEMI patients with obstructive sleep apnea (OSA) had a higher incidence of relative ST resolution <50% at 30 min after successful PCI than those without OSA [21]. Given that the patient does not sleep in the 30 min between PCI and measurement of resolution of ST deviation, an immediate effect of SDB on ST resolution is unlikely. The findings of the current study extend those of Nakashima et al., in that they represent novel data on resolution of ST deviation in patients with and without SDB in the first days after PCI with exposure to SDB before the measurements of ST deviation.
The differences in resolution of ST deviation seen in STEMI patients with and without SDB in the first 24 h after PCI were similar to those reported by Nakashima and colleagues 30 min after PCI. More than 24 h after PCI, residual ST deviation remained significantly higher in SDB patients. Prolonged time to resolution of ST deviation after STEMI is associated with a worse prognosis [7]. Almost complete resolution of ST deviation, a favorable prognostic marker [7, 8], was very rare in SDB patients in this study, both within 24 and more than 24 h after PCI. Because AHI is an independent predictor of the extent of residual ST deviation more than 24 h after PCI, it is possible that early treatment of SDB in the very first nights after STEMI may improve resolution of ST deviation and lead to a more favorable prognosis.
It has been shown that the extent of early ST segment resolution is a good predictor of the final size of the necrotic area in infarcted myocardium [9]. In this study, residual ST deviation more than 24 h after PCI correlated well with final infarct size measured using CMR 12 weeks after STEMI, whereas there was no correlation between final infarct size and earlier measurements of ST deviation. These data suggest that an important proportion of the negative impact of SDB on final infarct size occurs early, in the first days after STEMI.
After acute myocardial infarction, the presence of MVO inhibits myocardial healing and LV remodeling [31, 32]. The higher frequency of MVO within a few days after successful reperfusion in patients with SDB in this study supports the suggestion that SDB has a negative impact on damaged myocardium very early after STEMI.
Natriuretic peptides are markers of heart failure [33]. However, an early increase of NT-proBNP levels also reflects acute myocardial ischemia in patients with acute coronary syndrome [34, 35], and correlates with area at risk [36] and predicts ST segment resolution [37]. Higher levels of NT-proBNP in patients with SDB after STEMI have already been observed [38]. In this study, patients with SDB also had significantly higher serum levels of NT-proBNP within the first week after STEMI. This suggests prolonged myocardial ischemia in patients with SDB in the first days after myocardial infarction.
Pathophysiological considerations
Prompt reperfusion therapy is the key to a favorable prognosis in acute myocardial infarction [2], but does not overcome all the factors that can negatively influence the damaged myocardium. The prolonged resolution of ST deviation and greater extent of MVO in STEMI patients with SDB documented in this study might be caused by an increased mismatch of oxygen demand and supply. SDB causes increased sympathetic activation, arousals from sleep, and intermittent hypoxia, associated with acute surges in heart rate, blood pressure, and LV afterload [39]. In patients without cardiac disease and early after acute myocardial infarction, 24-hour blood pressure, heart rate, and cardiac workload, and thus myocardial oxygen demand [40, 41], are significantly increased in the presence of SDB [17, 42]. In addition, mechanical stress caused by negative intrathoracic pressures during nocturnal apnea episodes may also increase myocardial oxygen demand [39]. Increased oxygen demand is paralleled by reduced oxygen supply to damaged myocardial cells during nocturnal apneas and hypopneas which lead to intermittent hypoxia. It has been demonstrated that an obstructive apnea is associated with an 8‑second delay between an increase in myocardial workload and the associated increase in coronary blood flow [43], and that SDB can impair myocardial tissue perfusion in STEMI patients [21]. Coronary vasodilatation as a physiological reaction to myocardial hypoxia is reduced by endothelial dysfunction [44] and arteriosclerosis [45], both of which are significantly associated with the severity of SDB [46, 47]. The apnea-related mismatch of oxygen demand and supply may be the crucial hit to cells in the myocardium at risk of infarction in the first days immediately after STEMI. This may lead to a larger final extent of necrotic myocardium and to more severe heart failure.
Study limitations
Differences in resolution of ST deviation and the extent of MVO in STEMI patients with and without SDB were striking, but these findings have to be interpreted in the light of the following limitations. Because of the observational study design, the results can only show that there is an association between SDB and less resolution of ST deviation and more MVO in the first days after STEMI, but it is not possible to establish a cause-and-effect relationship. In addition, the sample size in this study was small.
Conclusion
This study documented prolonged resolution of ST deviation and a greater extent of MVO after STEMI in patients with SDB. The present findings suggest that SDB-related myocardial ischemia on the myocardium at risk takes place in the first days after STEMI. This raises the question of whether prompt diagnosis of unknown SDB and early treatment after STEMI may prevent further myocardial damage. Such questions are currently being investigated in an ongoing multicenter randomized controlled trial of adaptive servoventilation in patients with acute myocardial infarction and SDB (TEAM-ASV; NCT02093377).
Change history
23 May 2018
Erratum to:
Somnologie 2018
https://doi.org/10.1007/s11818-018-0154-8
The article “Resolution of ST deviation after myocardial infarction in patients with and without sleep-disordered breathing”, written by Ulrich Sterz, Stefan Buchner, Andrea Hetzenecker, Anna Satzl, Kurt Debl, Andreas Luchner, …
Abbreviations
- AHI:
-
Apnea–hypopnea index
- CMR:
-
Cardiac magnetic resonance imaging
- ECG:
-
Electrocardiogram
- LV:
-
Left ventricular
- MVO:
-
Microvascular obstruction
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- PCI:
-
Percutaneous coronary intervention
- SD:
-
Standard deviation
- SDB:
-
Sleep-disordered breathing
- STEMI:
-
ST elevation myocardial infarction
References
Wu E, Ortiz JT, Tejedor P et al (2008) Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: prospective cohort study. Heart 94:730–736
Keeley EC, Boura JA, Grines CL (2003) Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 361:13–20
Van de Werf F, Bax J, Betriu A et al (2008) Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 29:2909–2945
O’Regan DP, Ahmed R, Neuwirth C et al (2009) Cardiac MRI of myocardial salvage at the peri-infarct border zones after primary coronary intervention. Am J Physiol Heart Circ Physiol 297:H340–6
Maroko PR, Libby P, Covell JW, Sobel BE, Ross J, Braunwald E (1972) Precordial S‑T segment elevation mapping: an atraumatic method for assessing alterations in the extent of myocardial ischemic injury. The effects of pharmacologic and hemodynamic interventions. Am J Cardiol 29:223–230
Persson E, Pettersson J, Ringborn M et al (2006) Comparison of ST-segment deviation to scintigraphically quantified myocardial ischemia during acute coronary occlusion induced by percutaneous transluminal coronary angioplasty. Am J Cardiol 97:295–300
Schröder R, Dissmann R, Brüggemann T et al (1994) Extent of early ST segment elevation resolution: a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol 24:384–391
Buller CE, Fu Y, Mahaffey KW et al (2008) ST-segment recovery and outcome after primary percutaneous coronary intervention for ST-elevation myocardial infarction: insights from the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. Circulation 118:1335–1346
Dizon JM, Brener SJ, Maehara A et al (2014) Relationship between ST-segment resolution and anterior infarct size after primary percutaneous coronary intervention: analysis from the INFUSE-AMI trial. Eur Heart J Acute Cardiovasc Care 3:78–83
Wu KC, Zerhouni EA, Judd RM et al (1998) Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97:765–772
Buchner S, Greimel T, Hetzenecker A et al (2012) Natural course of sleep-disordered breathing after acute myocardial infarction. Eur Respir J 40:1173–1179
Ludka O, Stepanova R, Vyskocilova M et al (2014) Sleep apnea prevalence in acute myocardial infarction—the Sleep Apnea in Post-acute Myocardial Infarction Patients (SAPAMI) study. Int J Cardiol 176:13–19
Steiner S, Arzt M (2014) Koronare Herzkrankheit und schlafbezogene Atmungsstörungen. Somnologie (Berl) 18:189–193
Peled N, Abinader EG, Pillar G, Sharif D, Lavie P (1999) Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol 34:1744–1749
Franklin KA, Nilsson JB, Sahlin C, Näslund U (1995) Sleep apnoea and nocturnal angina. Lancet 345:1085–1087
Hung J, Whitford EG, Parsons RW, Hillman DR (1990) Association of sleep apnoea with myocardial infarction in men. Lancet 336:261–264
Hetzenecker A, Buchner S, Greimel T et al (2013) Cardiac workload in patients with sleep-disordered breathing early after acute myocardial infarction. Chest 143:1294–1301
Nakashima H, Katayama T, Takagi C et al (2006) Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur Heart J 27:2317–2322
Buchner S, Eglseer M, Debl K et al (2014) Sleep disordered breathing and enlargement of the right heart after myocardial infarction. Eur Respir J 45(3):680–690. https://doi.org/10.1183/09031936.00057014
Buchner S, Satzl A, Debl K et al (2014) Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J 35:192–199
Nakashima H, Muto S, Amenomori K, Shiraishi Y, Nunohiro T, Suzuki S (2011) Impact of obstructive sleep apnea on myocardial tissue perfusion in patients with ST-segment elevation myocardial infarction. Circ J 75:890–896
Matetzky S, Novikov M, Gruberg L et al (1999) The significance of persistent ST elevation versus early resolution of ST segment elevation after primary PTCA. J Am Coll Cardiol 34:1932–1938
Kushner FG, Hand M, Smith SC et al (2009) 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 54:2205–2241
Iber C (2007) AASM manual for the scoring of sleep and associated events: rules, terminology and technical. American Academy of Sleep Medicine, Westchester
Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667–89.
Brownfield J, Herbert M (2008) EKG criteria for fibrinolysis: what’s up with the J point? West J Emerg Med 9:40–42
O’Donnell T, Dikici E, Setser R, White RD (2005) Tracking and analysis of cine-delayed enhancement MR. Med Image Comput Comput Assist Interv 8:692–700
de van’t Hof LG, Arnoud WJ, de Boer M et al (2004) Time-to-treatment significantly affects the extent of ST-segment resolution and myocardial blush in patients with acute myocardial infarction treated by primary angioplasty. Eur Heart J 25:1009–1013
de Lemos JA, Braunwald E (2001) ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J Am Coll Cardiol 38:1283–1294
Eitel I, Desch S, Fuernau G et al (2010) Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol 55:2470–2479
Wu KC (2012) CMR of microvascular obstruction and hemorrhage in myocardial infarction. J Cardiovasc Magn Reson 14:68
Ørn S, Manhenke C, Greve OJ et al (2009) Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur Heart J 30:1978–1985
Palazzuoli A, Gallotta M, Quatrini I, Nuti R (2010) Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. Vasc Health Risk Manag 6:411–418
Buchner S, Debl K, Barlage S et al (2010) Dynamic changes in N‑terminal pro-brain natriuretic peptide in acute coronary syndromes treated with percutaneous coronary intervention: a marker of ischemic burden, reperfusion and outcome. Clin Chem Lab Med 48:875–881
Nikolaou NI, Kyriakides ZS, Tsaglis EP, Antonatos DG, Kartsagoulis EC, Tsigas DL (2005) Early brain natriuretic peptide increase reflects acute myocardial ischemia in patients with ongoing chest pain. Int J Cardiol 101:223–229
Ndrepepa G, Braun S, Mehilli J et al (2006) N‑terminal pro-brain natriuretic peptide on admission in patients with acute myocardial infarction and correlation with scintigraphic infarct size, efficacy of reperfusion, and prognosis. Am J Cardiol 97:1151–1156
Peng B, Xia H, Ni A, Wu G, Jiang X (2015) Serum NT-proBNP bei Aufnahme kann Rückbildung der ST-Hebung bei Patienten mit akutem Herzinfarkt nach primärer perkutaner Koronarintervention vorhersagen. Herz 40(6):898–905
Zhang W, Sun Y, Li T, Zhang G, Wang Y, Sun H (2012) The effect of obstructive sleep apnea-hypopnea syndrome on acute myocardial infarction. Bratisl Lek Listy 113:565–568
Tkacova R, Rankin F, Fitzgerald FS, Floras JS, Bradley TD (1998) Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation 98:2269–2275
Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y (1972) Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol 32:516–522
Baller D, Bretschneider HJ, Hellige G (1979) Validity of myocardial oxygen consumption parameters. Clin Cardiol 2:317–327
Davies CWH (2000) Case-control study of 24 h ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax 55:736–740
Hamilton GS, Meredith IT, Walker AM, Solin P (2009) Obstructive sleep apnea leads to transient uncoupling of coronary blood flow and myocardial work in humans. Sleep 32:263–270
Hamilton GS, Solin P, Walker A (2008) Coronary blood flow becomes uncoupled from myocardial work during obstructive sleep apnea in the presence of endothelial dysfunction. Sleep 31:809–816
Arbab-Zadeh A, Levine BD, Trost JC et al (2009) The effect of acute hypoxemia on coronary arterial dimensions in patients with coronary artery disease. Cardiology 113:149–154
Schulz R, Seeger W, Fegbeutel C et al (2005) Changes in extracranial arteries in obstructive sleep apnoea. Eur Respir J 25:69–74
Kadohira T, Kobayashi Y, Iwata Y, Kitahara H, Komuro I (2011) Coronary artery endothelial dysfunction associated with sleep apnea. Angiology 62:397–400
Acknowledgements
The authors thank Astrid Brandl-Novak, Astrid Braune, Ruth Luigart, and Katja Ziczinski for excellent assistance. English language editing assistance was provided by Nicola Ryan. This assistance was funded by ResMed.
Funding
The study was funded by ResMed (Martinsried, Germany), Philips Home Healthcare Solutions (Murrysville, PA, USA), and the Faculty of Medicine University of Regensburg, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Arzt receives grant support from ResMed (Martinsried, Germany), Philips Home Healthcare Solutions (Murrysville, PA, USA), and the German Foundation for Cardiac Research (Deutsche Stiftung für Herzforschung). M. Arzt is the holder of an endowed professorship from the Free State of Bavaria at the University of Regensburg that was donated by ResMed (Martinsried, Germany) and Philips Home Healthcare Solutions (Murrysville, PA, USA). M. Arzt has previously received lecture fees from Philips Home Healthcare Solutions (Murrysville, PA, USA) and ResMed (Martinsried, Germany). U. Sterz, S. Buchner, A. Hetzenecker, A. Satzl, K. Debl, A. Luchner, O. Husser, O.W. Hamer, C. Fellner, F. Zeman, and L.S. Maier declare that they have no competing interests.
The local institutional ethics committee reviewed and approved the study protocol, and all research was carried out in accordance with Good Clinical Practice and the principles outlined in the 1964 Declaration of Helsinki and its later amendments. All patients provided written informed consent before enrolment in the study.
Additional information
The original version of this article was revised due to a retrospective Open Access order.
The following authors contributed equally: Ulrich Sterz and Stefan Buchner.
This observational study was performed at the Universitätsklinikum Regensburg.
Caption Electronic Supplementary Material
11818_2018_154_MOESM1_ESM.tif
Fig. S1. Flow of patients through the study. AHI apnea–hypopnea index; CMR cardiac magnetic resonance; ECG electrocardiogram; NSTEMI non-ST elevation myocardial infarction; PCI percutaneous coronary intervention; PSG polysomnography; SDB sleep-disordered breathing; STEMI ST elevation myocardial infarction
11818_2018_154_MOESM2_ESM.tif
Fig. S2. Relative resolution of ST elevation 0–24 h (a) or >24 h (b) after PCI by the presence of sleep-disordered breathing
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Sterz, U., Buchner, S., Hetzenecker, A. et al. Resolution of ST deviation after myocardial infarction in patients with and without sleep-disordered breathing. Somnologie 23, 8–16 (2019). https://doi.org/10.1007/s11818-018-0154-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11818-018-0154-8