Abstract
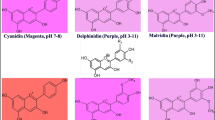
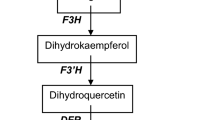
Anthocyanins are synthesized via the flavonoid pathway through a complex expression of several genes such as MYB transcription factors. Anthocyanins protect plants against biotic and abiotic stresses. Herein, we studied the effect of expression of MYB (VvmybA1 cloned from the red grape and Ruby cloned from ‘Moro’ blood orange) transcription factors in “Mexican” lime on juice quality and leaf pigments, leaf metabolites, and phytohormones. Anthocyanins, furanocoumarins, flavonoids, and hydroxycinnamates were analyzed with high-performance liquid chromatography–mass spectrometry, whereas chlorophylls, carotenoids, and xanthophylls were analyzed using HPLC coupled with photodiode array detector (PDA). The rest of metabolites were analyzed using gas chromatography–mass spectrometry. Overexpression of VvmybA1 and Ruby resulted in accumulation of anthocyanins in leaves, flowers, and fruits of the transgenic plants. However, the level of anthocyanins in Ruby plants was significantly lower than that in VvmybA1 plants. The level of anthocyanins and the gene expression of VvmybA1 and Ruby in young leaves were higher than mature leaves. On the other hand, the level of several furanocoumarins, and hydroxycinnamates decreased in mature VvmybA1 leaves, indicating a drainage of p-coumaric acid due to the induction of anthocyanins biosynthesis. The level of chlorophyll decreased in mature VvmybA1 leaves, whereas zeaxanthin level increased, indicated a photoprotection role for anthocyanins. Most of polar and volatile metabolites also decreased VvmybA1 leaves, indicating a decrease in the photosynthetic efficiency. Benzoic acid and salicylic acid increased, whereas auxins decreased. The level of abscisic acid was not affected by the overexpression of VvmybA1 and the plants showed normal growth and development. Overexpression of VvmybA1 highly increased the antioxidant activity of the transgenic juice and leaves, whereas overexpression of Ruby showed only a slight increase. The pH, °Brix value, and TA of the transgenic juice were not affected by the expression of VvmybA1 or Ruby.






Similar content being viewed by others
References
Acosta-Estrada BA, Serna-Saldívar SO, Gutiérrez-Uribe JA (2015) Chemopreventive effects of feruloyl putrescines from wastewater (Nejayote) of lime-cooked white maize (Zea mays). J Cereal Sci 64:23–28
Arimura G-i, Matsui K, Takabayashi J (2009) Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 50:911–923
Baldwin IT (2010) Plant volatiles. Curr Biol 20:R392–R397
Bartley GE, Scolnik PA (1995) Plant carotenoids: pigments for photoprotection, visual attraction, and human health. Plant Cell 7:1027–1038
Bennett RN, Wallsgrove RM (1994) Secondary metabolites in plant defence mechanisms. New Phytol 127:617–633
Bradley JM, Davies KM, Deroles SC, Bloor SJ, Lewis DH (1998) The maize Lc regulatory gene up-regulates the flavonoid biosynthetic pathway of Petunia. Plant J 13:381–392
Bridle P, Timberlake CF (1997) Anthocyanins as natural food colours—selected aspects. Food Chem 58:103–109
Brouillard R (1982) Chemical structure of anthocyanins. In: Markakis P (ed) Anthocyanins as food colors. Academic Press, New York, pp 1–40
Burger J, Edwards GE (1996) Photosynthetic efficiency, and photodamage by UV and visible radiation, in red versus green leaf coleus varieties. Plant Cell Physiol 37:395–399
Butelli E, Licciardello C, Zhang Y, Liu J, Mackay S, Bailey P et al (2012) Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 24:1242–1255
De Beer D, Joubert E, Gelderblom WCA, Manley M (2003) Antioxidant activity of south African red and white cultivar wines: free radical scavenging. J Agric Food Chem 51:902–909
Deighton N, Brennan R, Finn C, Davies HV (2000) Antioxidant properties of domesticated and wild Rubus species. J Sci Food Agric 80:1307–1313
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta Bioenerg 1020:1–24
Dixon RA, Liu C, Jun JH (2013) Metabolic engineering of anthocyanins and condensed tannins in plants. Curr Opin Biotechnol 24:329–335
Du H, Wu N, Chang Y, Li X, Xiao J, Xiong L (2013) Carotenoid deficiency impairs ABA and IAA biosynthesis and differentially affects drought and cold tolerance in rice. Plant Mol Biol 83:475–488
Dutt M, Grosser JW (2010) An embryogenic suspension cell culture system for Agrobacterium-mediated transformation of citrus. Plant Cell Rep 29:1251–1260
Dutt M, Stanton D, Grosser JW (2016) Ornacitrus: Development of genetically modified anthocyanin-expressing citrus with both ornamental and fresh fruit potential. J Am Soc Hortic Sci 141:54–61
Edelenbos M, Christensen LP, Grevsen K (2001) HPLC determination of chlorophyll and carotenoid pigments in processed green pea cultivars (Pisum sativum L.). J Agric Food Chem 49:4768–4774
Fabroni S, Ballistreri G, Amenta M, Romeo FV, Rapisarda P (2016) Screening of the anthocyanin profile and in vitro pancreatic lipase inhibition by anthocyanin-containing extracts of fruits, vegetables, legumes and cereals. J Sci Food Agric 96:4713–4723
Gould KS, Markham KR, Smith RH, Goris JJ (2000) Functional role of anthocyanins in the leaves of Quintinia serrata A. Cunn. J Exp Bot 51:1107–1115
Gould KS, Vogelmann TC, Han T, Clearwater MJ (2002) Profiles of photosynthesis within red and green leaves of Quintinia serrata. Physiol Plant 116:127–133
Hijaz F, Killiny N (2014) Collection and chemical composition of phloem sap from Citrus sinensis L. Osbeck (sweet orange). PLoS One 9(7):e101830
Hijaz F, El-Shesheny I, Killiny N (2013) Herbivory by the insect Diaphorina citri induces greater change in citrus plant volatile profile than does infection by the bacterium, Candidatus Liberibacter asiaticus. Plant Signal Behav 8(10):e25677
Jung S (2004) Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol Biochem 42:225–231
Karageorgou P, Manetas Y (2006) The importance of being red when young: anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiol 26:613–621
Killiny N, Nehela Y (2017) One target, two mechanisms: The impact of ‘Candidatus Liberibacter asiaticus’ and its vector, Diaphorina citri, on citrus leaf pigments. Mol Plant Microbe Interact 30:543–556
Lazebnik J, Frago E, Dicke M, van Loon JJA (2014) Phytohormone mediation of interactions between herbivores and plant pathogens. J Chem Ecol 40:730–741
Lee HS (2002) Characterization of major anthocyanins and the color of red-fleshed Budd Blood orange (Citrus sinensis). J Agric Food Chem 50:1243–1246
Li H, Flachowsky H, Fischer TC, Hanke M-V, Forkmann G, Treutter D et al (2007) Maize Lc transcription factor enhances biosynthesis of anthocyanins, distinct proanthocyanidins and phenylpropanoids in apple (Malus domestica Borkh.). Planta 226:1243–1254
Lila MA (2004) Anthocyanins and human health: An in vitro investigative approach. J Biomed Biotechnol 2004:306–313
Liu Y, Heying E, Tanumihardjo SA (2012) History, global distribution, and nutritional importance of citrus fruits. Compr Rev Food Sci Food Saf 11:530–545
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Mano Y, Nemoto K (2012) The pathway of auxin biosynthesis in plants. J Exp Bot 63:2853–2872
Milenković SM, Zvezdanović JB, Anđelković TD, Marković DZ (2012) The identification of chlorophyll and its derivatives in the pigment mixtures: HPLC-chromatography, visible and mass spectroscopy studies. Adv Technol 1:16–24
Morton JF (1987) Mexican lime. In: Morton JF (ed) Fruits of warm climates. Julia F. Morton, Miami, pp 168–172
Mouly PP, Gaydou EM, Lapierre L, Corsetti J (1999) Differentiation of several geographical origins in single-strength Valencia orange juices using quantitative comparison of carotenoid profiles. J Agric Food Chem 47:4038–4045
Murray JR, Hackett WP (1991) Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L. Plant Physiol 97:343–351
Naz S, Siddiqi R, Ahmad S, Rasool SA, Sayeed SA (2007) Antibacterial activity directed isolation of compounds from Punica granatum. J Food Sci 72:M341–M345
Nehela Y, Hijaz F, Elzaawely AA, El-Zahaby HM, Killiny N (2016) Phytohormone profiling of the sweet orange (Citrus sinensis (L.) Osbeck) leaves and roots using GC–MS-based method. J Plant Physiol 199:12–17
Neill SO, Gould KS (2003) Anthocyanins in leaves: light attenuators or antioxidants? Funct Plant Biol 30:865–873
Penjor T, Mimura T, Matsumoto R et al (2014) Characterization of limes (Citrus aurantifolia) grown in Bhutan and Indonesia using high-throughput sequencing. Sci Rep 4:4853
Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A (2013) Plant flavonoids-biosynthesis, transport and involvement in stress responses. Int J Mol Sci 14:14950–14973
Pietrini F, Massacci A (1998) Leaf anthocyanin content changes in Zea mays L. grown at low temperature: significance for the relationship between the quantum yield of PS II and the apparent quantum yield of CO2 assimilation. Photosynth Res 58:213–219
Qiu J, Sun S, Luo S, Zhang J, Xiao X, Zhang L, Wang F, Liu S (2014) Arabidopsis AtPAP1 transcription factor induces anthocyanin production in transgenic Taraxacum brevicorniculatum. Plant Cell Rep 33:669–680
Rau W, Schrott EL (1987) Blue light control of pigment biosynthesis. In: Senger H (ed) The blue light responses: phenomena and occurrence in plants and microorganisms, vol 1. CRC Press, Boca Raton, pp 43–63
Reddy AM, Reddy VS, Scheffler BE, Wienand U, Reddy AR (2007) Novel transgenic rice overexpressing anthocyanidin synthase accumulates a mixture of flavonoids leading to an increased antioxidant potential. Metab Eng 9:95–111
Rodrigo M-J, Marcos JF, Zacarías L (2004) Biochemical and molecular analysis of carotenoid biosynthesis in flavedo of orange (Citrus sinensis L.) during fruit development and maturation. J Agric Food Chem 52:6724–6731
Sanchez-Moreno C (2002) Review: methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int 8:121–137
Steyn WJ, Wand SJE, Holcroft DM, Jacobs G (2002) Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytol 155:349–361
Stover E, Avila Y, Li ZT, Gray D (2013) Transgenic expression in citrus of Vitis MybA1 from a bidirectional promoter resulted in variable anthocyanin expression and was not suitable as a screenable marker without antibiotic selection. Proc Fla State Hortic Soc 126:1–5
Studham ME, MacIntosh GC (2012) Multiple phytohormone signals control the transcriptional response to soybean aphid infestation in susceptible and resistant soybean plants. Mol Plant Microbe Interact 26:116–129
Takos AM, Jaffé FW, Jacob SR, Bogs J, Robinson SP, Walker AR (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142:1216–1232
Tsuda T, Shiga K, Ohshima K, Kawakishi S, Osawa T (1996) Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem Pharmacol 52:1033–1039
Tsuda T, Horio F, Uchida K, Aoki H, Osawa T (2003) Dietary cyanidin 3-O-beta-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr 133:2125–2130
Wang H, Cao G, Prior RL (1997) Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem 45:304–309
Wei X, Chen C, Yu Q, Gady A, Yu Y, Liang G, Gmitter FG (2014a) Comparison of carotenoid accumulation and biosynthetic gene expression between Valencia and Rohde Red Valencia sweet oranges. Plant Sci 227:28–36
Wei X, Chen C, Yu Q, Gady A, Yu Y, Liang G, Gmitter FG (2014b) Novel expression patterns of carotenoid pathway-related genes in citrus leaves and maturing fruits. Tree Genet Genomes 10:439–448
Wiczkowski W, Szawara-Nowak D, Topolska J (2013) Red cabbage anthocyanins: profile, isolation, identification, and antioxidant activity. Food Res Int 51:303–309
Xu Z-S, Feng K, Que F, Wang F, Xiong A-S (2017) A MYB transcription factor, DcMYB6, is involved in regulating anthocyanin biosynthesis in purple carrot taproots. Sci Rep 7:45324
Yang J, Guo J, Yuan J (2008) In vitro antioxidant properties of rutin. LWT Food Sci Technol 41:1060–1066
Zanatta CF, Cuevas E, Bobbio FO et al (2005) Determination of Anthocyanins from Camu-camu (Myrciaria dubia) by HPLC–PDA, HPLC–MS, and NMR. J Agric Food Chem 53:9531–9535
Acknowledgements
The authors acknowledge our CREC colleagues for the helpful discussion. We thank Laina Lindsey and Floyd Butz for maintaining the trees in greenhouses.
Author information
Authors and Affiliations
Contributions
NK conceived the research; FH performed the HPLC analysis for anthocyanins, antioxidant activity, and juice quality; YN performed the HPLC analyses for chlorophyll, carotenoid, and xanthophyll, and GC–MS analyses for phytohormones; SEJ performed the GC–MS analyses for primary and secondary metabolites and volatiles; JAM performed the LC–MS analysis for flavonoids; MD and JWG provided the plants (plants were produced in a previous study); NK and FH performed theoretical research; FH, YN, SEJ, JAM, and NK analyzed data; FH and NK wrote the first draft of manuscript; and FH, YN, SEJ, MD, JWG, JAM, and NK finalized the paper.
Corresponding author
Rights and permissions
About this article
Cite this article
Hijaz, F., Nehela, Y., Jones, S.E. et al. Metabolically engineered anthocyanin-producing lime provides additional nutritional value and antioxidant potential to juice. Plant Biotechnol Rep 12, 329–346 (2018). https://doi.org/10.1007/s11816-018-0497-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-018-0497-4