Abstract
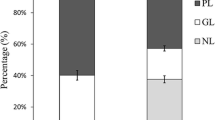
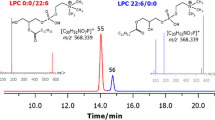
A fast and efficient shotgun lipidomics strategy was applied to analyze phospholipids (PL) in the oyster Crassostrea plicatula, including 29 species of phosphatidylcholine (PtdCho), 23 species of phosphatidylethanolamine (PtdEtn), 11 species of phosphatidylserine (PtdSer), 6 species of phosphatidylinositol (PtdIns), and 17 species of lysophospholipids (Lyso-PL). During storage at 4 °C for 7 days, the PL content decreased by 68.08%, but a significant increase in the FFA content was observed (from 63.11 to 318.72 μg/g). PtdCho and PtdIns decreased relatively by 64.97 and 67.49%, and PtdSer decreased most markedly by 74.15%. However, the PtdEtn content increased slightly during the early stages of storage but subsequently began to decrease. Moreover, PL with eicosapentaenoic acid (EPA-PL) and docosahexaenoic acid (DHA-PL) decreased by 51.77 and 50.61%, whereas plasmalogens were relatively stable showing only a 25.46% decrease. In particular, through enzyme activity analysis of lipase, phospholipase A1 (PLA1), phospholipase A2 (PLA2), phospholipase C (PLC), and phospholipase D (PLD), it was observed that the activities of all these enzymes increased at the early stage at 4 °C, but their activities were at lower levels when the oysters were stored at −20 °C. During the storage period at 4 °C, correlation analysis suggests that the degradation of PtdCho was mostly correlated to PLA2 (p < 0.05), whereas PtdEtn and PtdSer were more markedly correlated to lipase and PLD, respectively. The above result indicates that the hydrolysis mechanism of PL during seafood storage was correlated to the lipid hydrolytic enzyme activities under different storage temperatures.







Similar content being viewed by others
Change history
13 November 2017
Erratum to: Lipids https://doi.org/10.1007/s11745-017-4305-7
Abbreviations
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FFA:
-
Free (unesterified) fatty acids
- GL:
-
Glycolipid
- Lyso-PL:
-
Lysophospholipids
- Lyso-PtdCho:
-
Lysophosphatidylcholine
- NL:
-
Neutral lipids
- PakCho:
-
1-O-Alkyl-2-acyl-sn-glycero-3-phosphocholine
- PakSer:
-
1-O-Alkyl-2-acyl-sn-glycero-3-phosphoserine
- PL:
-
Phospholipids
- PLA1 :
-
Phospholipase A1
- PLA2 :
-
Phospholipase A2
- PLC:
-
Phospholipase C
- PLD:
-
Phospholipase D
- PlsEtn:
-
Plasmalogen
- PtdCho:
-
Phosphatidylcholine
- PtdEtn:
-
Phosphatidylethanolamine
- PtdIns:
-
Phosphatidylinositol
- PtdSer:
-
Phosphatidylserine
References
Yu H, Li Q, Yu R (2008) Microsatellite variation in the oyster Crassostrea plicatula along the coast of China. J Ocean Univ China 7:432–438
Linehan LG, O’Connor TP, Burnell G (1999) Seasonal variation in the chemical composition and fatty acid profile of Pacific oysters (Crassostrea gigas). Food Chem 64:211–214
Pernet F, Gauthier-Clerc S, Mayrand E (2007) Change in lipid composition in eastern oyster (Crassostrea virginica Gmelin) exposed to constant or fluctuating temperature regimes. Comp Biochem Phys B 147:557–565
Le GF, Kraffe E, Marty Y, Donaghy L, Soudant P (2011) Membrane phospholipid composition of hemocytes in the Pacific oyster Crassostrea gigas and the Manila clam Ruditapes philippinarum. Comp Biochem Phys A 159:383–391
Chen S, Belikova NA, Subbaiah PV (2012) Structural elucidation of molecular species of Pacific oyster ether amino phospholipids by normal-phase liquid chromatography/negative-ion electrospray ionization and quadrupole/multiple-stage linear ion-trap mass spectrometry. Anal Chim Acta 735:76–89
Hanuš LO, Levitsky DO, Shkrob I, Dembitsky VM (2009) Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem 116:491–498
Brosche TM, Haase K, Sieber C, Bertsch T (2007) Decreased plasmalogen concentration as a surrogate marker of oxidative stress in patients presenting with acute coronary syndromes or supraventricular tachycardias. Clin Chem Lab Med 45:689–691
Pourashouri P, Chapela MJ, Atanassova M, Cabado AG, Vieites JM, Aubourg SP (2013) Quality loss assessment in fish-based ready-to-eat foods during refrigerated storage. Grasas Aceites 64:22–29
Ravandi A, Leibundgut G, Hung MY, Patel M, Hutchins PM, Murphy RC, Prasad A, Mahmud E, Miller YI, Dennis EA (2014) Release and capture of bioactive oxidized phospholipids and oxidized cholesteryl esters during percutaneous coronary and peripheral arterial interventions in humans. J Am Coll Cardiol 63:1961–1971
Torkamani AE, Juliano P, Ajlouni S, Singh TK (2014) Impact of ultrasound treatment on lipid oxidation of Cheddar cheese whey. Ultrason Sonochem 21:951–957
Shewfelt RL, Mcdonald RE, Hultin HO (2010) Effect of phospholipid hydrolysis on lipid oxidation in flounder muscle microsomes. J Food Sci 46:1297–1301
Motilva MJ, Toldrá F, Flores J (1992) Assay of lipase and esterase activities in fresh pork meat and dry-cured ham. Eur Food Res Techn 195:446–450
Smichi N, Gargouri Y, Miled N, Fendri A (2013) A grey mullet enzyme displaying both lipase and phospholipase activities: purification and characterization. Int J Biol Macromol 58:87–94
Kurtovic I, Marshall SN, Xin Z, Simpson BK (2009) Lipases from mammals and fishes. Rev Fish Sci 17:18–40
Chowdhury HH, Rebolj K, Kreft M, Zorec R, Maček P, Sepčić K (2008) Lysophospholipids prevent binding of a cytolytic protein ostreolysin to cholesterol-enriched membrane domains. Toxicon 51:1345–1356
Tokumura A (2011) Physiological significance of lysophospholipids that act on the lumen side of mammalian lower digestive tracts. J Health Sci 57:115–128
Nishikawa Y, Furukawa A, Shiga I, Muroi Y, Ishii T, Hongo Y, Takahashi S, Sugawara T, Koshino H, Ohnishi M (2015) Cytoprotective effects of lysophospholipids from sea cucumber Holothuria atra. PLoS One 10:e0135701
Zhao PY, Li HL, Hossain MM, Kim IH (2015) Effect of emulsifier (lysophospholipids) on growth performance, nutrient digestibility and blood profile in weanling pigs. Anim Feed Sci Tech 207:190–195
Ali AH, Zou X, Lu J, Abed SM, Yao Y, Tao G, Jin Q, Wang X (2017) Identification of phospholipids classes and molecular species in different types of egg yolk by using UPLC-Q-TOF-MS. Food Chem 221:58–66
Shen Q, Wang Y, Gong L, Guo R, Dong W, Cheung HY (2012) Shotgun lipidomics strategy for fast analysis of phospholipids in fisheries waste and its potential in species differentiation. J Agric Food Chem 60:9384–9393
Maqsood S, Abushelaibi A, Manheem K, Kadim IT (2015) Characterisation of the lipid and protein fraction of fresh camel meat and the associated changes during refrigerated storage. J Food Compos Anal 41:212–220
Lowry RR, Tinsley IJ (1976) Rapid colorimetric determination of free fatty acids. J Am Oil Chem Soc 53:470–472
Costa DdSV, Bragagnolo N (2017) Development and validation of a novel microwave assisted extraction method for fish lipids. Eur J Lipid Sci Tech 119:UNSP1600108
Rached F, Santos RD, Camont L, Miname MH, Lhomme M, Dauteuille C, Lecocq S, Serrano C Jr, Chapman MJ, Kontush A (2014) Defective functionality of HDL particles in familial apoA-I deficiency: relevance of alterations in HDL lipidome and proteome. J Lipid Res 55:2509–2520
Smichi N, Fendri A, Zarai Z, Bouchaala E, Chérif S, Gargouri Y, Miled N (2012) Lipolytic activity levels and colipase presence in digestive glands of some marine animals. Fish Physiol Biochem 38:449–1458
Wang H, Zhong S, Ma H, Zhang J, Qi W (2012) Screening and characterization of a novel alkaline lipase from Acinetobacter calcoaceticus 1–7 isolated from Bohai Bay in China for detergent formulation. Braz J Microbiol 43:148–156
Qiu S, Lai L (2013) Tailoring the pH dependence of human non-pancreatic secretory phospholipase A2 by engineering surface charges. App Biochem Biotech 171:1454–1464
Clark GC, Briggs DC, Karasawa T, Wang X, Cole AR, Maegawa T, Jayasekera PN, Naylor CE, Miller J, Moss DS (2003) Clostridium absonum alpha-toxin: new insights into clostridial phospholipase C substrate binding and specificity. J Mol Biol 333:759–769
Huang Y, Wu X, Ahmad QI, Chen H (1997) Determination of phosphatidyl choline specific phospholipase D using enzyme coupling colorimetric method and its application. J Shanghai Med 24:343–346
Aaraas R, Hernar IJ, Vorre A, Bergslien H, Lunestad BT, Skeie S, Slinde E, Mortensen S (2004) Sensory, histological, and bacteriological changes in flat oysters, Ostrea edulis L., during different storage conditions. J Food Sci 69:S205–S210
Songsaeng S, Sophanodora P, Kaewsrithong J, Ohshima T (2010) Quality changes in oyster (Crassostrea belcheri) during frozen storage as affected by freezing and antioxidant. Food Chem 123:286–290
Songsaeng S, Sophanodora P, Kaewsrithong J, Ohshima T (2009) Changes in lipids of white-scar oyster (Crassostrea belcheri) during chilled storage under different salt concentrations. As J Food Ag-Ind 2009:140–154
Pogoda B, Buck BH, Saborowski R, Hagen W (2013) Biochemical and elemental composition of the offshore-cultivated oysters Ostrea edulis and Crassostrea gigas. Aquaculture 400–401:53–60
Shen YN, Zhang DL, Zhang B, Jiang S, Liu BS, Huang GJ, Yu DH (2016) A baseline study on lipid and fatty acid composition in the pearl oyster, Pinctada fucata. Aquacult Int 24:523–536
Zhao YD, Wang M, Lindström ME, Li JB (2015) Fatty acid and lipid profiles with emphasis on n-3 fatty acids and phospholipids from Ciona intestinalis. Lipids 50:1009–1027
Boselli E, Pacetti D, Lucci P, Frega NG (2012) Characterization of phospholipid molecular species in the edible parts of bony fish and shellfish. J Agric Food Chem 60:3234–3245
Rodriguez-Cuenca S, Pellegrinelli V, Campbell M, Oresic M, Vidal-Puig A (2017) Sphingolipids and glycerophospholipids—the “ying and yang” of lipotoxicity in metabolic diseases. Prog Lipid Res 66:14–29
Delaporte M, Chu FL, Langdon C (2007) Changes in biochemical and hemocyte parameters of the Pacific oysters Crassostrea gigas fed T-Iso supplemented with lipid emulsions rich in eicosapentaenoic acid. J Exp Mar Biol Ecol 343:261–275
Liu CC, Lu JZ, Su YC (2009) Effects of flash freezing, followed by frozen storage, on reducing Vibrio parahaemolyticus in Pacific raw oysters (Crassostrea gigas). J Food Protect 72:174–177
Stanley DW (1991) Biological membrane deterioration and associated quality losses in food tissues. Criti Rev Food Sci 30:487–553
Selvy PE, Lavieri RR, Lindsley CW, Brown HA (2011) Phospholipase D: enzymology, functionality, and chemical modulation. Chem Rev 111:6064–6119
Alan NH, Graeme TC, George SA, Anthony DP (2001) Highly saturated endonuclear phosphatidylcholine is synthesized in situ and colocated with CDP-choline pathway enzymes. J Biol Chem 276:8492–8849
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31330060).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s11745-017-4308-4.
About this article
Cite this article
Chen, Q., Wang, X., Cong, P. et al. Mechanism of Phospholipid Hydrolysis for Oyster Crassostrea plicatula Phospholipids During Storage Using Shotgun Lipidomics. Lipids 52, 1045–1058 (2017). https://doi.org/10.1007/s11745-017-4305-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4305-7