Abstract
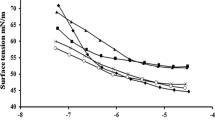
Two sugar-based ester quaternary ammonium compounds were synthesized and their surface behavior and antimicrobial activity were investigated. The compatibility with anionic surfactant sodium dodecyl sulfate (SDS) was further studied. Results indicated that the synthesized cationic surfactants showed high surface activity and good synergistic effects with SDS.




Similar content being viewed by others
References
Egan PA (1989) Surfactants from biomass. Chemtech 19:758–762
Holmberg K (2001) Natural surfactants. Curr Opin Colloid Interface Sci 6:148–159
Rubingh D, Holland PM (1990) Cationic surfactants. Marcel Dekker, New York
Greek BF (1991) Sales of detergents growing despite recession. Chem Eng News 69:25–52
Regulation (EC) No. 648/2004 of the European parliament and the council of 31 March 2004 on detergents. Off J Eur Union, 8.4.2004, L 104/1–35
Hill K, Rhode O (1999) Sugar-based surfactants for consumer products and technical applications. Fett/Lipid 101:25–33
Lichtenthaler FW (2007) Carbohydrates as renewable raw materials: a major challenge of green chemistry. In: Tundo P, Perosa A, Zecchini F (eds) Methods and reagents for green chemistry. Wiley, Hoboken, pp 23–63
Patel M (2003) Surfactants based on renewable raw materials. J Ind Ecol 7:47–62
Bazito RC, Seoud OAE (2001) Sugar-based cationic surfactants: synthesis and aggregation of methyl 2-acylamido-6-trimethylammonio-2,6-dideoxy-d-glucopyranoside chlorides. J Surfactant Deterg 4:395–400
Abudureheman W, Abudurexiti A, Liu C, Li YP (2010) Progress with respect to synthesis of sugar-based ionic surfactants. China Surfactant Deterg Cosmet 40:278–286
Soussan E, Mille C, Blanzat M, Bordat P, Rico-Lattes I (2008) Sugar-derived tricatenar catanionic surfactant: synthesis, self-assembly properties, and hydrophilic probe encapsulation by vesicles. Langmuir 24:2326–2330
Negm NA, Mohamed AS (2008) Synthesis, characterization, and biological activity of sugar-based gemini cationic amphiphiles. J Surfactant Deterg 11:215–221
Plat T, Linhardt RJ (2001) Synthesis and applications of sucrose-based esters. J Surfactant Deterg 4:415–421
von Rybinski W, Hill K (1998) Alkyl polyglycosides—properties and applications of a new class of surfactants. Angew Chem Int Ed 37:1328–1345
Burczykn B (2003) Novel saccharide-based surfactants. In: Holmberg K (ed) Novel surfactants: preparation, applications, and biodegradability. Marcel Dekker, New York, pp 129–192
Goclik V, Mischnick P (2003) Determination of the DS and substituent distribution of cationic alkyl polyglycosides and cationic starch ethers by GLC after dealkylation with morpholine. Carbohydr Res 338:733–741
Khan A, Marques E (1996) Catanionic surfactants. In: Robb ID (ed) Specialist surfactants. Springer, Netherlands, pp 37–80
Zou LH, Fang Y, Lu SS (2001) Mixing behaviors and application of anionic-cationic surfactants. China Surfactant Deterg Cosmet 31:37–40
Minor RS (1919) Physical measurements: a laboratory manual in general physics for colleges. Part II: heat, mechanics and properties of matter. Berkeley, New York, pp 23–26
Heiks JR, Barnett MK, Jones LV, Orban E (1954) The density, surface tension and viscosity of deuterium oxide at elevated temperatures. J Phys Chem 58:488–491
Hou RS (1994) General physics experiment. Chengdu University of Science and Technology Press, Chengdu
Bikerman JJ (1953) Foams: theory and industrial application. Reinhold, New York, pp 38–47
Liu ZD, Liang PL, Chen XB, Yu JX (2007) Synthesis and characterization of sugar-based quaternary ammonium salt surfactants. Fine Chem 24:870–877
Cooper KE (1972) The theory of antibiotic inhibition zones. In: Kavanagh F (ed) Analytical microbiology, vol 2. Academic Press, New York, pp 13–30
Liang SN, Duan ZF, Fu L, Huang DX, Liu QY (2005) Comparative research on the antibacterial action of several types of cinnamic acid derivative and cinnamic acid. Food Sci Technol 9:71–73
Wang JT, Wang WX, Wang FS, Du ZP (2009) Surface activity of blend systems based on glucosidyl quaternary ammonium salt and alpha-olefin sulfonate. China Surfactant Deterg Cosmet 39:162–165
Wang JT, Wang WX, Wang FS, Du ZP, Song YB (2009) Study on properties of mixed systems based on glucoside quaternary and sodium dodecyl sulfate. Chem Res Appl 21:344–348
Acknowledgments
The authors are grateful to the Department of Education of Anhui Province (KJ2012A236) for the support of this study. We thank Dr. Zhi-feng Zhu for his kind assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gan, C., Wang, H., Zhao, Z. et al. Sugar-Based Ester Quaternary Ammonium Compounds and Their Surfactant Properties. J Surfact Deterg 17, 465–470 (2014). https://doi.org/10.1007/s11743-014-1562-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1562-9