Abstract
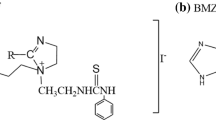
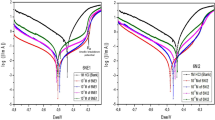
The inhibition performance of two imidazoline inhibitors, 1-(2-thioureaethyl)-2-alkyl-imidazoline (TAI) and chloride-1-(2,3-dihydroxylpropyl)-1-(2-thioureaethyl)-2-alkyl-imidazoline sodium phosphate (TAIP), for Q235 steel in saltwater saturated with CO2 was studied by using molecular dynamics simulations and quantum chemistry calculations. The conclusions were experimentally verified by weight loss, polarization curves, electrochemical impedance spectroscopy (EIS) and surface analysis techniques. The theoretical results suggest that imidazoline ring and heteroatoms are the active site and the adsorption stability weakens gradually in the order of TAIP, TAI. Experimental results show that the two inhibitors act as mixed type inhibitors and can inhibit the corrosion of Q235 in CO2 saturated saltwater solution.




Similar content being viewed by others
References
Lopez DA, Schreiner WH, De Sanchez SR, Simison SN (2004) The influence of inhibitors molecular structure and steel microstructure on corrosion layers in CO2 corrosion: an XPS and SEM characterization. Appl Surf Sci 236:77–79
Durnie WH, Kinsella BJ, De Marco R, Jefferson A (2001) A study of the adsorption properties of commercial carbon dioxide corrosion inhibitor formulations. J Appl Electrochem 31:1221–1226
Liu X, Okafor PC, Zheng YG (2009) The inhibition of CO2 corrosion of N80 mild steel in single liquid phase and liquid/particle two-phase flow by aminoethyl imidazoline derivatives. Corros Sci 51:744–751
Shukla SK, Quraishi MA, Prakash R (2008) A self-doped conducting polymer “polyanthranilic acid”: an efficient corrosion inhibitor for mild steel in acidic solution. Corros Sci 50:2867–2872
Aiad IA, Hafiz AA, El-Awady MY, Habib AO (2010) Some imidazoline derivatives as corrosion inhibitors. J Surf Deterg 13:247–254
Palomar ME, Olivares-Xometl CO, Likhanova NV, Perez-Navarrete JB (2011) Imidazolium, pyridinium and dimethyl-ethylbenzyl ammonium derived compounds. J Surf Deterg 14:211–220
Kesavan D, Tamizh MM, Gopiraman M, Sulochana N, Karvembu R (2012) Physicochemical studies of 4-substituted n-(2-mercaptophenyl)-salicylideneimines: corrosion inhibition of mild steel in an acid medium. J Surf Deterg 15:567–576
Gece G, Bilgic S (2009) Quantum chemical study of some cyclic nitrogen compounds as corrosion inhibitors of steel in NaCl media. Corros Sci 51:1876–1878
Gece G (2008) The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci 50:2981–2992
Arslan T, Kandemirli F, Ebenso EE, Love L, Alemu H (2009) Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros Sci 51:35–47
Khalil N (2003) Quantum chemical approach of corrosion inhibition. Electrochim Acta 48:2635–2640
Khaled KF (2011) Experimental and computational investigations of corrosion and corrosion inhibition of iron in acid solutions. J Appl Electrochem 41:277–287
Khaled KF (2008) Molecular simulation, quantum chemical calculations and electrochemical studies for inhibition of mild steel by triazoles. Electrochim Acta 53:3484–3492
Tang Y, Yang X, Yang W, Chen Y, Wan R (2010) Experimental and molecular dynamics studies on corrosion inhibition of mild steel by 2-amino-5-phenyl-1,3,4- thia-diazo. Corros Sci 52:242–249
Karelson M, Lobanov VS (1996) Quantum-chemical. descriptors in QSAR/QSPR Studies. Chem Rev 96:1027–1043
Mulliken RS (1955) Electronic population analysis on LCAO-MO molecular wave functions. I J Chem Phys 23:1833–1841
Issa RM, Awad MK, Atlam FM (2008) Quantum chemical studies on the inhibition of corrosion of copper surface by substituted uracils. Appl Surf Sci 255:2433–2441
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1873
Jamalizadeh E, Hosseini SMA, Jafari AH (2009) Quantum chemical studies on corrosion inhibition of some lactones on mild steel in acid media. Corros Sci 51:1428–1435
Özcan M, Karadağ F, Dehri I (2008) Investigation of adsorption characteristics of methionine at mild steel/sulfuric acid interface: an experimental and theoretical study. Coll Surf A 316:55–61
Lavrich DJ, Wetterer SM, Bernasek SL, Scoles G (1998) Physisorption and chemisorption of alkanethiols and alkyl sulfides on Au(111). J Phys Chem B 102:3456–3465
Hu SQ, Hu JC, Fan CC (2010) Theoretical and experimental study of corrosion inhibition performance of new imidazoline corrosion inhibitors. Acta Chim Sinica 68:2051–2058
Wang B, Du M, Zhang J, Gao CJ (2011) Electrochemical and surface analysis studies on corrosion inhibition of Q235 steel by imidazoline derivative against CO2 corrosion. Corros Sci 53:353–361
Wang B, Du M, Zhang J (2009) Study of the inhibition mechanism of imidazoline derivative inhibitor on CO2 corrosion for Q235 steel. Adv Materials Res 79–82:981–984
Lopez DA, Simison SN, De Sanchez SR (2005) Inhibitors performance in CO2 corrosion: eIS studies on the interaction between their molecular structure and steel microstructure. Corros Sci 47:735–755
Khaled KF, Hackerman N (2003) Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 1 M HCl solutions. Electrochim Acta 48:2715–2723
Nesic S, Postlethwaite J, Olsen S (1996) An electrochemical model for prediction of corrosion of mild steel in aqueous carbon dioxide solutions. Corrosion 52:280–306
Gojic M (2001) The effect of propargylic alcohol on the corrosion inhibition of low alloy CrMo steel in sulphuric acid. Corros Sci 43:919–929
El-Tabei AS, Hegazy MA (2013) A corrosion inhibition study of a novel synthesized gemini nonionic surfactant for carbon steel in 1 M HCl solution. J Surf Deterg. doi:10.1007/s11743-013-1457-1
Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Project No. 40806030).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zhang, J., Niu, L., Zhu, F. et al. Theoretical and Experimental Studies for Corrosion Inhibition Performance of Q235 Steel by Imidazoline Inhibitors against CO2 Corrosion. J Surfact Deterg 16, 947–956 (2013). https://doi.org/10.1007/s11743-013-1515-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-013-1515-8