Abstract
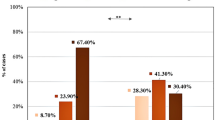
This study aims at assessing NF-kB activity in unstable angina (UA) patients free of symptoms after a 1 year follow-up (1YFU). Plasma oxidized low-density lipoproteins (oxLDL), circulating NF-kB, Interleukin 6 (IL-6) and Interleukin 1β (IL-1β), high-sensitivity C-reactive protein (hs-CRP), as markers of oxidative stress and inflammation and plasma double-stranded DNA (ds-DNA), as marker of Neutrophil Extracellular Traps (NETs), were measured in 23 of the previously enrolled 27 UA patients. These measurements were compared to the UA data at baseline, and then compared to the data derived from the stable angina (SA) and controls (C) enrolled in our previous study (we demonstrated that UA had higher levels of NF-kB compared to SA and C). After a 1YFU, UA patients show a significant decrease in NF-kB, IL-6, hs-CRP, oxLDL, and ds-DNA plasma levels (p < 0.001) and in IL-1β and White Blood Cells (WBC) (p < 0.005), without differences in lipid and glucose assessment. If compared to SA and C, UA after a 1YFU have higher levels of NF-kB, IL-6, ds-DNA, WBC, and oxLDL compared to C (p < 0.001), but only IL-6 is higher than SA (p < 0.001). No differences are found in lipid and glucose assessment. After a 1YFU, patients with a history of UA improve their oxidative and inflammatory status, such as the levels of circulating ds-DNA, without achieving the status of C. They become comparable to SA subjects. This study provides new insight on the multiple and apparently contradictory facets of NF-kB in UA and on its possible role as mediator in NETs’ formation.



Similar content being viewed by others
References
Mathers CD, Loncar D (2006) Projection of global mortality and burden of disease from 2002 to 2030. PLoS Med 3:2011–2030. https://doi.org/10.1371/journal.pmed.0030442
Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 37:267–315. https://doi.org/10.1093/eurheartj/ehv320
Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, Venge P, Hasin Y, Galvani M, Koenig W, Hamm C, Alpert JS, Katus H, Jaffe AS (2012) How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 33:2252–2257. https://doi.org/10.1093/eurheartj/ehs154
Braunwald E, Morrow DA (2013) Unstable angina: is it time for a requiem? Circulation 127:2452–2457. https://doi.org/10.1161/CIRCULATIONAHA.113.001258
Mockel M, Searle J, Hamm C, Slagman A, Blankenberg S, Huber K, Katus H, Liebetrau C, Muller C, Muller R, Peitsmeyer P, von Recum J, Tajsic M, Vollert JO, Giannitsis E (2015) Early discharge using single cardiac troponin and copeptin testing in patients with suspected acute coronary syndrome (ACS): a randomized, controlled clinical process study. Eur Heart J 36:369–376. https://doi.org/10.1093/eurheartj/ehu178
Kumar A, Takada Y, Borick AM, Aggarwal BB (2004) Nuclear factor kappa B: its role in health and disease. J Mol Med 82:434–448. https://doi.org/10.1007/s00109-004-0555-y
Mitchell S, Vargas J, Hoffmann A (2016) Signaling via the NF-kB system. Syst Biol Med 8:227–241. https://doi.org/10.1002/wsbm.1331
Hayden MS, Ghosh S (2008) Shared principles in NF-kappa B signaling. Cell 132:344–362. https://doi.org/10.1016/j.cell.2008.01.020
Gordon JW, Shaw JA, Kirshenbaum LA (2011) Multiple facets of NF-kB in the heart: to be or not to NF-kB. Circ Res 108:1122–1132. https://doi.org/10.1161/CIRCRESAHA.110.226928
Karin M, Lin A (2002) NF-kB at the crossroads of life and death. Nat Immun 3:221–227. https://doi.org/10.1038/ni0302-221
Suzuki N, Kamataki A, Yamaki J, Homma Y (2008) Characterization of circulating DNA in healthy human plasma. Clin Chim Acta 387:55–59. https://doi.org/10.1016/j.cca.2007.09.001
Swarup V, Rajeswari A (2007) Circulating (cell-free) nucleic acids—a promising non-invasive tool for early detection of several human diseases. FEBS Lett 581:795–799. https://doi.org/10.1016/j.febslet.2007.01.051
Cui M, Fan M, Jing R, Wang H, Qin J, Sheng H, Wang Y, Wu X, Zhang L, Zhu J, Ju S (2013) Cell-free circulating DNA: a new biomarker for the acute coronary syndrome. Cardiology 124(2):76–84. https://doi.org/10.1159/000345855
Chang CP, Chia RH, Wu TL, Tsao KC, Sun CF, Wu JT (2003) Elevated cell-free serum DNA detected in patients with myocardial infarction. Clin Chim Acta 327(1–2):95–101
Destouni A, Vrettou C, Antonatos D, Chouliaras G, Traeger-Synodinos J, Patsilinakos S, Kitsiou-Tzeli S, Tsigas D, Kanavakis E (2009) Cell-free DNA levels in acute myocardial infarction patients during hospitalization. Acta Cardiol 64(1):51–57. https://doi.org/10.2143/AC.64.1.2034362
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535. https://doi.org/10.1126/science.1092385
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176(2):231–241. https://doi.org/10.1083/jcb.200606027
Marcos V, Zhou Z, Yildirim AO, Bohla A, Hector A, Vitkov L, Wiedenbauer E-M, Krautgartner WD, Stoiber W, Belohradsky BH (2010) CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med 16:1018–1023. https://doi.org/10.1038/nm.2209
Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, Piconese S, Parenza M, Guiducci C, Vitali C (2012) Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 120:3007–3018. https://doi.org/10.1182/blood-2012-03-416156
Brinkmann V, Zychlinsky A (2012) Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 198:773–783. https://doi.org/10.1083/jcb.201203170
Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD (2012) Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA 109:13076–13081. https://doi.org/10.1073/pnas.1200419109
Demers M, Wagner DD (2014) NETosis: a new factor in tumour progression and cancer-associated thrombosis. Semin Thromb Haemost 40(3):277–283. https://doi.org/10.1055/s-0034-1370765
Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L, Crijns HJ, Wagner DD, Kietselaer BL (2013) Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 33(8):2032–2040. https://doi.org/10.1161/ATVBAHA.113.301627
Megens RT, Vijayan S, Lievens D, Döring Y, van Zandvoort MA, Grommes J, Weber C, Soehnlein O (2012) Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost 107(3):597–598. https://doi.org/10.1160/TH11-09-0650
De Boer OJ, Li X, Teeling P, Mackaay C, Ploegmakers HJ, van der Loos CM, Daemen MJ, de Winter RJ, van der Wal AC (2013) Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb Haemost 109(2):290–297. https://doi.org/10.1160/TH12-06-0425
Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenböck A, Simon D, Laimer D, Bangert C, Kammerlander A, Mascherbauer J, Winter MP, Distelmaier K, Adlbrecht C, Preissner KT, Lang IM (2015) Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 116(7):1182–1192. https://doi.org/10.1161/CIRCRESAHA.116.304944
Mozzini C, Garbin U, Fratta Pasini AM, Cominacini L (2016) An exploratory look at NETosis in atherosclerosis. Intern Emerg Med. https://doi.org/10.1007/s11739-016-1543-2
Cominacini L, Anselmi M, Garbin U, Fratta Pasini A, Stranieri C, Fusaro M, Nava C, Agostoni P, Keta D, Zardini P, Sawamura T, Lo Cascio V (2005) Enhanced plasma levels of oxidized low-density lipoprotein increase circulating nuclear factor-kappa B activation in patients with unstable angina. J Am Coll Cardiol 46:799–806. https://doi.org/10.1016/j.jacc.2005.05.063
Fratta Pasini AM, Anselmi M, Garbin U, Franchi E, Stranieri C, Nava MC, Boccioletti V, Vassanelli L, Cominacini L (2007) Enhanced levels of oxidized low density lipoprotein prime monocytes to cytokine overproduction via upregulation of CD14 and Toll-like receptor 4 in unstable angina. Arterioscler Thromb Vasc Biol 27:1991–1997. https://doi.org/10.1161/ATVBAHA.107.142695
Lindorfer MA, Schuman TA, Craig ML, Martin EN, Taylor RP (2001) A bispecific dsDNA monoclonal antibody construct for clearance of anti-dsDNA IgG in systemic lupus erythematosus. J Immunol Methods 248(1–2):125–138
Li Y, Ha T, Gao X, Kelley J, Williams DI, Browder IW, Kao RI, Li C (2004) NF-kB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol 287:H1712–H1721. https://doi.org/10.1152/ajpheart.00124.2004
Frantz S, Hu K, Bayer B, Gerondakis S, Strottmann J, Adamek A, Ertl G, Bauersachs J (2006) Absence of NF-kB subunit p50 improves heart failure after myocardial infarction. FASEB J 20:1918–1920. https://doi.org/10.1096/fj.05-5133fje
Dhingra R, Shaw JA, Aviv Y, Kirshenbaum LA (2010) Dichotomous actions of NF-kappaB signaling pathways in heart. J Cardiovasc Transl Res 4:344–354. https://doi.org/10.1007/s12265-010-9195-5
You M, Ku PT, Hrdlikova R, Bose HR (1997) Ch-IAP-1 member of the inhibitor of apoptosis protein family is a mediator of the antiapoptotic activity of the v-Rel oncoprotection. Mol Cell Biol 17:7328–7341
Chu ZL, Mc Kinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW (1997) Suppression of tumour necrosis factor induced death by inhibitor of apoptosis c-IAP2 is under NF-kB control. Proc Natl Acad Sci 94:10057–10062
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS (1998) NF-kB antiapoptosis: induction of TRAF1 and TRAF2 and cIAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680–1683
Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, Dean K, Franzoso G (2006) The NF- kB- mediated control of JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ 13:712–729. https://doi.org/10.1038/sj.cdd.4401865
Beere HM (2004) The stress of dying: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651. https://doi.org/10.1242/jcs.01284
Beere HM (2005) Death versus survival: functional interaction between the apoptotic and stress-inducible heat shock protein pathways. J Clin Investig 115:2633–2639. https://doi.org/10.1172/JCI26471
Valen G, Hansson GK, Dumitrescu A, Vaage J (2000) Unstable angina activates myocardial heat shock protein 72, endothelial nitric oxide synthase and transcription factor NF kappa B and AP-1. Cardiovasc Res 47:49–56
Czibik G, Wu Z, Berne G, Tarkka M, Vaage J, Laurikka J, Jarvinen O, Valen G (2008) Human adaptation to ischemia by preconditioning or unstable angina: involvement of nuclear factor kappa B, but not hypoxia-inducible factor 1 alpha in the heart. Eur J Cardiothorac Surg 34:976–984. https://doi.org/10.1016/j.ejcts.2008.07.066
Tahepold P, Vaage J, Starkopt J, Valen G (2003) Hyperoxia elicits myocardial protection through a nuclear factor kappa B-dependent mechanism in the rat heart. J Thorac Cardiovasc Surg 125:650–660. https://doi.org/10.1067/mtc.2003.36
Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, Qlu Y, Li JJ, Bolli R (1999) Nuclear factor kappa B plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res 84:1095–1109
Misra A, Haudek SB, Knuefermann P, Vallejo JG, Chen ZJ, Michael LH, Sivasubramanian N, Olson EN, Entman ML, Mann DI (2003) Nuclear factor kappa B protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation 108:3075–3078. https://doi.org/10.1161/01.CIR.0000108929.93074.0B
Libby P (2013) Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 368:2004–2013. https://doi.org/10.1056/NEJMra1216063
Buffon A, Basucci LM, Liuzzo G (2002) Widespread coronary inflammation in unstable angina. N Engl J Med 347:5–12. https://doi.org/10.1056/NEJMoa012295
Bogaty P, Boyer L, Simard S, Dauwe F, Dupuis R, Verret B, Huynh T, Betrand F, Dagenais GR, Brophy JM (2008) The RISCA (Recurrence and Inflammation in the Acute Coronary Syndromes) Study. Clinical utility of C-reactive protein measured ad admission, hospital discharge and 1 month later to predict outcome in patients with acute coronary syndrome. J Am Coll Cardiol 51(24):2339–2346. https://doi.org/10.1016/j.jacc.2008.03.019
Liuzzo G, Biasucci LM, Gallimore JR (1994) The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Eng J Med 331:417–424. https://doi.org/10.1056/NEJM199408183310701
Biasucci LM, Liuzzo G, Grillo RL (1999) Elevated levels of C-reactive protein at discharge in patients with unstable angina predict recurrent instability. Circulation 99:855–860
Liuzzo G, Santamaria M, Biasucci LM, Narducci M, Colafrancesco V, Porto A, Brugaletta S, Pinnelli M, Rizzello V, Maseri A, Crea F (2007) Persistent activation of Nuclear Factor Kappa-B signaling pathway in patients with unstable angina and elevated levels of C-reactive protein. J Am Coll Cardiol 49:185–194. https://doi.org/10.1016/j.jacc.2006.07.071
Lapponi MJ, Carestia A, Landoni VI, Rivadeneyra L, Etulain J, Negrotto S, Pozner RG, Schattner M (2013) Regulation of neutrophil extracellular trap formation by anti-inflammatory drugs. J Pharmacol Exp Ther 345(3):430–437. https://doi.org/10.1124/jpet.112.202879
Yin MJ, Yamamoto Y, Gaynor RB (1998) The anti-inflammatory agent aspirin and salicylate inhibit the activity of IkB kinase beta. Nature 396:77–80. https://doi.org/10.1038/23948
Mc Donald PP, Bald A, Cassatella MA (1997) Activation of the NF-kappaB pathway by inflammatory stimuli in human neutrophils. Blood 89:3421–3433
Massberg S, Grahl L, von Bruehl ML (2010) Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 16:887–896. https://doi.org/10.1038/nm.2184
Ross R (1999) Atherosclerosis: an inflammatory disease. N Engl J Med 340:115–126
Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101:1767–1772
Libby P, Ridker PM, Hansson GK (2009) Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 54:2129–2138. https://doi.org/10.1016/j.jacc.2009.09.009
Biasucci LM, Vitelli A, Liuzzo G, Altamura S, Caligiuri G, Monaco C, Rebuzzi AG, Ciliberto G, Maseri A (1996) Elevated levels of Interleukin-6 in unstable angina. Circulation 94:874–877
Dinarello CA (2000) The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343:732–734. https://doi.org/10.1056/NEJM200009073431011
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550. https://doi.org/10.1146/annurev.immunol.021908.132612
Sims JE, Smith DE (2010) The IL-1 family: regulators of immunity. Nat Rev Immunol 10:89–102. https://doi.org/10.1038/nri2691
Stutz A, Golenbock DT, Latz E (2009) Inflammasomes: too big to miss. J Clin Invest 119:3502–3511. https://doi.org/10.1172/JCI40599
Ogura Y, Sutterwala FS, Flavell RA (2006) The inflammasome: first line of the immune response to cell stress. Cell 126:659–662. https://doi.org/10.1016/j.cell.2006.08.002
Rajama K, Lappalainen J, Oorni K (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5:e11765. https://doi.org/10.1371/journal.pone.0011765
Ridker P, Howard C, Walter V, Everett B, Libby P, Hensen J, Thuren T (2012) Effects of interleukin-1 inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 126:2739–2748. https://doi.org/10.1161/CIRCULATIONAHA.112.122556
Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V (2015) Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349(6245):316–320. https://doi.org/10.1126/science.aaa8064
Joshi MB, Lad A, Prasad AB, Balakrishnan A, Ramachandra L, Satyamoorthy K (2013) High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett 587:2241–2246. https://doi.org/10.1016/j.febslet.2013.05.053
Ikeda U, Ito T, Shimada K (2001) Interleukin-6 and acute coronary syndrome. Clin Cardiol 24(11):701–704
Rus HG, Vlaicu R, Niculescu F (1996) Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 127(2):263–271
Manten A, de Winter RJ, Minnema MC, ten Cate H, Lijmer JG, Adams R, Peters RJ, van Deventer SJ (1998) Procoagulant and proinflammatory activity in acute coronary syndromes. Cardiovasc Res 40(2):389–395
Wang XH, Liu SQ, Wang YL, Jin Y (2014) Correlation of serum high-sensitivity C-reactive protein and interleukin-6 in patients with acute coronary syndrome. Genet Mol Res 13(2):4260–4266
Lai CL, Ji YR, Liu XH, Xing JP, Zhao JQ (2011) Relationship between coronary atherosclerosis plaque characteristics and high sensitivity C-reactive proteins, interleukin-6. Chin Med J (Eng) 124(16):2452–2456
Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M (1995) Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Investig 96(6):2758–2767. https://doi.org/10.1172/JCI118345
Neumann FJ, Ott I, Marx N, Luther T, Kenngott S, Gawaz M, Kotzsch M, Schömig A (1997) Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol 17(12):3399–3405
Gwechenberger M, Mendoza LH, Youker KA, Frangogiannis NG, Smith CW, Michael LH, Entman ML (1999) Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation 99(4):546–551
Caselli C, De Graaf MA, Lorenzoni V, Rovai D, Marinelli M, Del Ry S, Giannessi D, Bax JJ, Neglia D, Schlte AJ (2015) HDL cholesterol, leptin and interleukin-6 predict high risk coronary anatomy assessed by CT angiography in patients with stable chest pain. Atherosclerosis 241(1):55–61. https://doi.org/10.1016/j.atherosclerosis.2015.04.811
Koyama K, Yoneyama K, Mitarai T, Ishibashi Y, Takahashi E, Kongoji K, Harada T, Akashi YJ (2015) Association between inflammatory biomarkers and thin-cap fibroatheroma detected by optical coherence tomography in patients with coronary heart disease. Arch Med Sci 11(3):505–512. https://doi.org/10.5114/aoms.2015.52352
Plutzki J (2001) Inflammatory pathways in atherosclerosis and acute coronary syndromes. Am J Cardiol 88(8A):10K–15K
Brasier AR (2010) The nuclear factor-kB—interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 86:211–218. https://doi.org/10.1093/cvr/cvq076
Libermann TA, Baltimore D (1990) Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol 10(5):2327–2334
Lippi G, Sanchis-Gomar F, Cervellin G (2015) Cell-free DNA for diagnosing myocardial infarction: not ready for prime time. Clin Chem Lab Med 53(12):1895–1901. https://doi.org/10.1515/cclm-2015-0252
Tonello S, Rizzia M, Migliario M, Rocchetti V, Renò F (2017) Low concentrations of neutrophil extracellular traps induce proliferation in human keratinocytes via NF-kB activation. J Dermatol Sci 88:110–116
Author information
Authors and Affiliations
Contributions
CM and LC conceived the study; GP statistically analyzed the data; AF and UG revised the data and the manuscript; GS and CS performed the experiments; CM wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that they have no conflict of interest.
Statement of human and animal rights
The study was conducted in accordance with the ethical standards laid down in the Helsinki Declaration of 1975 and its late amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Mozzini, C., Garbin, U., Stranieri, C. et al. Nuclear factor kappa B in patients with a history of unstable angina: case re-opened. Intern Emerg Med 13, 699–707 (2018). https://doi.org/10.1007/s11739-018-1885-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-018-1885-z