Abstract
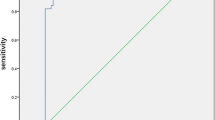
Pleural or abdominal effusions are frequent findings in ICU and Internal Medicine patients. Diagnostic gold standard to distinguish between transudate and exudate is represented by “Light’s Criteria,” but, unfortunately, the chemical–physical examination for their calculation is not a rapid test. Pursuing an acid–base assessment of the fluid by a blood-gas analyzer, an increase of lactate beyond the normal serum range is reported in the exudative effusions. The advantages of this test are that it is a very fast bed-side test, executable directly by the physician. The aim of this study is to evaluate whether the increase in lactate in pleural and abdominal effusions might be used as a criterion for the differential diagnosis of the nature of the fluid. Sixty-nine patients with pleural or abdominal effusions and clinical indication for thoracentesis or paracentesis were enrolled. Acid–base assessment with lactate, total protein, and LDH dosage on the serum, and acid–base assessment with lactate, total protein, and LDH dosage, cytology, and bacterial culture on the fluid were performed to each patient. Fluid–blood lactate difference (ΔLacFB) and fluid–blood lactate ratio (LacFB ratio) were calculated. A statistical analysis was carried out for fluid lactate (LacF), ΔLacFB, and LacFB ratio, performing ROC curves to find the cut-off values with best sensitivity (Sn) and specificity (Sp) predicting an exudate diagnosis: LacF: cut-off value: 2.4 mmol/L; AU-ROC 0.854 95% CI 0.756–0.952; Sn 0.77; Sp 0.84. ΔLacFB: cut-off value: 0.95 mmol/L; Au-ROC 0.876 95% CI 0.785–0.966; Sn 0.80; Sp 0.92. LacFB ratio: cut-off value: 2 mmol/L; Au-ROC 0.730 95% CI 0.609–0.851; Sn 0.74; Sp 0.65. Lactate dosage by blood-gas analyzer on pleural and abdominal effusions seems to be a promising tool to predict a diagnosis of exudate.


Similar content being viewed by others
References
Maskell NA, Butland RJA et al (2003) BTS guidelines for the investigation of an unilateral pleural effusion in adults. Thorax 58(Suppl II):ii8–ii17
Light RW, Mac Gregor MI, Luchsinger PC et al (1972) Pleural effusions; the diagnostic separation of transudates and exudates. Ann Intern Med 77:507–513
Light RW (2002) Pleural effusions. N Engl J Med 346(25):1971–1977
Wysenbeek AJ, Lahay M, Aelion JA et al (1985) Eosinophilic pleural effusions: a review of 36 cases. Respiration 48:73–76
Adelman M, Albelda SM, Gottlieb J et al (1984) Diagnostic utility of pleural fluid eosinophilia. Am J Med 77:915–920
Martinez-Garcia MA, Cases-Viedma E, Cordero-Rodriguez PJ et al (2000) Diagnostic utility of eosinophils in the pleural fluid. Eur Respir J 15:166–169
Levine H, Metzger W, Lacera D et al (1970) Diagnosis of tuberculous pleurisy by culture of pleural biopsy specimen. Arch Intern Med 126:269–271
Potts DE, Levin DC, Sahn SA (1976) Pleural fluid pH in parapneumonic effusions. Chest 70:328–331
Chavalittamrong B, Angsusingha K, Tuchinda M et al (1979) Diagnostic significance of pH, lactic acid dehydrogenase, lactate and glucose in pleural fluid. Respiration 38(2):112–120
Brumbaugh GW, Benson PA (1990) Partial pressures of oxygen and carbon dioxide, pH and concentrations of bicarbonate, lactate and glucose in pleural fluid from horses. Am J Vet Res 51(7):1032–1037
Rodriguez P, Lopez M (1989) Low glucose and pH levels in malignant pleural effusions. Diagnostic significance and prognostic value in respect to pleurodesis. Am Rev Respir Dis 139:663–667
Sahn SA (1985) Pathogenesis and clinical features of diseases associated with a low pleural fluid glucose. In: Chretien J, Bignon J, Hirsch A (eds) The pleura in health and disease. Marcel Dekker, New York, pp 267–285
Light RW, Ball WCJ (1973) Glucose and amylase in pleural effusions. JAMA 225:257–259
Potts DE, Willcox MA, Good JT Jr et al (1978) The acidosis of low-glucose pleural effusions. Am Rev Respir Dis 117:665–671
Ende N (1960) Studies of amylase activity in pleural effusions and ascites. Cancer 13:283–287
Sherr HP, Light RW, Merson MH et al (1972) Origin of pleural fluid amylase in esophageal rupture. Ann Intern Med 76:985–986
Kramer M (1989) High amylase levels in neoplasm-related pleural effusion. Ann Intern Med 110:567–569
Gil S, Martinez M, Cases V et al (1995) Pleural cholesterol in differentiating transudates and exudates. A prospective study of 232 cases. Respiration 62:57–63
Hamm H, Brohan U, Bohmer R et al (1987) Cholesterol in pleural effusions. A diagnostic aid. Chest 92:296–302
Ortega L, Heredia JL, Armengol R et al (1991) The differential diagnosis between pleural exudates and transudates: the value of cholesterol. Med Clin (Barc) 96:367–370
Nanji AA, Whitelow KJ (1984) Clinical utility of lactic acid measurement in body fluids other than plasma. J Emerg Med 1(6):521–526
Pettersson T, Ojala K, Weber TH (1985) Diagnostic significance of pleural fluid lactate concentrations. Infection 13(6):257–259
Gastrin B, Lovestad A (1988) Diagnostic significance of pleural fluid lactate concentration in pleural and pulmonary diseases. Scand J Infect Dis 20:85–90
Jokipii AM, Kiviranta K, Jokipii L (1987) Gas chromatographically quantitated lactate in empyema and other pleural effusions. Eur J Clin Microbiol 6(6):731–733
Brook I (1980) Measurement of lactic acid in pleural fluid. Respiration 40(6):344–348
Weynants P, Reynaert M, Lievens M et al (1987) Pleural fluid lactate in pleural effusion. Eur J Respir Dis 71(1):19–22
Brook I (1981) The importance of lactic acid levels in body fluids in the detection of bacterial infections. Rev Infect Dis 3(3):470–478
Bruun B, Stilbo I, Bartels P (1984) Value of pleural lactate in the differential diagnosis between empyema and non-bacterial pleural effusions. Acta Pathol Microbiol Immunol Scand B 92(2):85–88
Light RW, Erozan YS, Ball WCI (1973) Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med 132:854–860
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent J-L, Moreno R (2013) Surviving sepsis campaign guidelines committee including the pediatric subgroup: surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:N2
Kraut JA, Madias NE (2014) Lactic acidosis. N Engl J Med 371:2309–2319
Bar-Even A, Flamholz A, Noor E et al (2012) Rethinking glycolysis: on the biochemical logic of metabolic pathways. Nat Chem Biol 8(6):509–517
Meert KL, McCaulley L, Sarnaik AP (2012) Mechanism of lactic acidosis in children with acute severe asthma. Pediatr Crit Care Med 13(1):28–31
Kruse JA, Carlson RW (1987) Lactate metabolism. Crit Care Clin 3(4):725–746
Taylor DJ, Faragher EB, Evanson JM (1992) Inflammatory cytokines stimulate glucose uptake and glycolysis but reduce glucose oxidation in human dermal fibroblasts in vitro. Circ Shock 37:105–110
Suetrong B, Walley KR (2016) Lactic acidosis in sepsis: it’s not all anaerobic: implications for diagnosis and management. Chest 149(1):252–261
Suistomaa M, Ruokonen E, Kari A et al (2000) Time-pattern of lactate and lactate to pyruvate ratio in the first 24 hours of intensive care emergency admissions. Shock 14(1):8–12
Levy B, Desebbe O, Montemont C et al (2008) Increased aerobic glycolysis through beta2 stimulation is a common mechanism involved in lactate formation during shock states. Shock 30:417–421
Borregaard N, Herlin T (1982) Energy metabolism of human neutrophils during phagocytosis. J Clin Investig 70(3):550–557
Leverve XM, Mustafa I (2002) Lactate: a key metabolite in the intercellular metabolic interplay. Crit Care 6(4):284–285
Brooks GA (2009) Cell–cell and intracellular lactate shuttles. J Physiol 587(23):5591–5600
Brooks GA (2000) Intra- and extra-cellular lactate shuttles. Med Sci Sports Exerc 32(4):790–799
Smith SM, Eng RH, Campos JM et al (1989) d-lactic acid measurements in the diagnosis of bacterial infections. J Clin Microbiol 27(3):385–388
Wellmer A, Prange J, Gerber J (2001) Et al: d- and l-lactate in rabbit and human bacterial meningitis. Scand J Infect Dis 33(12):909–913
Xiong X, Yang Z, Liwei Z, Peng K, Nan J (2016) The diagnostic value of cerebrospinal fluid lactate for post-neurosurgical bacterial meningitis: a meta-analysis. BMC Infect Dis 16:483
Burgess LJ, Reuter H, Cartstens ME et al (2002) Cytokine production in patients with tuberculous pericarditis. Int J Tuberc Lung Dis 6:439
Hoefs JC (1983) Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med 102:260
Hoefs JC (1984) Ascitic fluid analysis in the differentiation of spontaneous bacterial peritonitis from gastrointestinal tract perforation into ascitic fluid. Hepatology 4:447
Hoefs JC (1981) Increase in ascites white blood cell and protein concentrations during diuresis in patients with chronic liver disease. Hepatology 1:249
Akriviadis EA, Runyon BA (1990) Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology 98:127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
This research was performed in two Emergency Departments and in an Internal Medicine Department of Southern Italy: Emergency Department “A. Cardarelli” Hospital, Naples—Emergency Department “San Paolo” Hospital, Naples—Internal Medicine and Hepatology Department, Second University of Naples, Naples.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Porta, G., Numis, F.G., Rosato, V. et al. Lactate determination in pleural and abdominal effusions: a quick diagnostic marker of exudate—a pilot study. Intern Emerg Med 13, 901–906 (2018). https://doi.org/10.1007/s11739-017-1757-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-017-1757-y