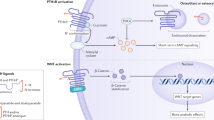
Abstract
Osteoporosis is characterized by low bone mass and qualitative structural abnormalities of bone tissue, leading to increased bone fragility that results in fractures. Pharmacological therapy is aimed at decreasing the risk of fracture, mainly correcting the imbalance between bone resorption and formation at the level of bone remodeling units. Anabolic therapy has the capability to increase bone mass to a greater extent than traditional antiresorptive agents. The only currently available drug licensed is parathyroid hormone 1–34 (teriparatide); new drugs are on the horizon, targeting the stimulation of bone formation, and therefore improving bone mass, structure and ultimately skeletal strength. These are represented by abaloparatide (a 34-amino acid peptide which incorporates critical N-terminal residues, shared by parathyroid hormone and parathyroid hormone-related protein, followed by sequences unique to the latter protein) and romosozumab (an antibody to sclerostin). In the future, the availability of new anabolic treatment will allow a more extensive utilization of additive and sequential approach, with the goal of both prolonging the period of treatment and, more importantly, avoiding the side effects consequent to long-term use of traditional drugs.

(data are redrawn from Tarantino U)

Similar content being viewed by others
References
Papapoulos SE (2015) Anabolic bone therapies in 2014: new bone-forming treatments for osteoporosis. Nat Rev Endocrinol 11:69–70. doi:10.1038/nrendo.2014.214
Freemantle N, Cooper C, Roux C et al (2010) Baseline observations from the POSSIBLE EU® study: characteristics of postmenopausal women receiving bone loss medications. Arch Osteoporos 5:61–72. doi:10.1007/s11657-010-0035-7
Holloway KL, Henry MJ, Brennan-Olsen SL et al (2016) Non-hip and non-vertebral fractures: the neglected fracture sites. Osteoporos Int 27:905–913. doi:10.1007/s00198-015-3322-8
Hernlund E, Svedbom A, Ivergård M et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1–2):136. doi:10.1007/s11657-013-0136-1
Romagnoli E, Carnevale V, Nofroni I et al (2004) Quality of life in ambulatory postmenopausal women: the impact of reduced bone mineral density and subclinical fractures. Osteoporos Int 15:975–980. doi:10.1007/s00198-004-1633-2
Romagnoli E, Carnevale V, Calandra P et al (2003) Impact of fractures on health care in a major university hospital in Rome. Aging Clin Exp Res 15:505–511. doi:10.1007/BF03327374
Kanis JA, Cooper C, Rizzoli R et al (2017) Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int 28(7):2023–2034. doi:10.1007/s00198-017-4009-0
Salked G, Cameron ID, Cumming RG et al (2000) Quality of life related to fear of falling and hip fracture in older women: a time trade off study. Br Med J 320(7231):341–346
Pfeifer M, Sinaki M, Geusens P et al (2004) Musculoskeletal rehabilitation in osteoporosis: a review. J Bone Miner Res 19:1208–1214. doi:10.1359/JBMR.040507
Boonen S, Lips P, Bouillon R et al (2007) Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J Clin Endocrinol Metab 92(4):1415–1423. doi:10.1210/jc.2006-1404
Romagnoli E, Pepe J, Piemonte S et al (2013) Management of endocrine disease: value and limitations of assessing vitamin D nutritional status and advised levels of vitamin D supplementation. Eur J Endocrinol 169:59–69. doi:10.1530/EJE-13-0435
Cipriani C, Pepe J, Piemonte S et al (2014) Vitamin D and its relationship with obesity and muscle. Int J Endocrinol. doi:10.1155/2014/841248
Murad MH, Drake MT, Mullan RJ et al (2012) Comparative Effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab 97(6):1871–1880. doi:10.1210/jc.2011-3060
Riggs BL, Parfitt AM (2005) Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 20:177–184. doi:10.1359/JBMR.041114
Minisola S (2014) Romosozumab: from basic to clinical aspects. Expert Opin Biol Ther 14(9):1225–1228. doi:10.1517/14712598.2014.920815
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Eng J Med 344(19):1434–1441. doi:10.1056/NEJM200105103441904
Dempster DW, Zhou H, Recker RR et al (2016) A longitudinal study of skeletal histomorphometry at 6 and 24 months across four bone envelopes in postmenopausal women with osteoporosis receiving teriparatide or zoledronic acid in the SHOTZ trial. J Bone Miner Res 31:1429–1439. doi:10.1002/jbmr.2804
Moreira CA, Dempster DW (2017) Histomorphometric changes following treatment for osteoporosis. J Endocrinol Invest. doi:10.1007/s40618-017-0662-6
Hansen KE, Wilson HA, Zapalowski C et al (2011) Uncertainties in the prevention and treatment of glucocorticoid-induced osteoporosis. J Bone Miner Res 26:1989–1996. doi:10.1002/jbmr.362
Mazziotti G, Formenti AM, Adler RA et al (2016) Glucocorticoid-induced osteoporosis: pathophysiological role of GH/IGF-I and PTH/VITAMIN D axes, treatment options and guidelines. Endocrine 54:603–611. doi:10.1007/s12020-016-1146-8
Farahmand P, Marin F, Hawkins F et al (2013) Early changes in biochemical markers of bone formation during teriparatide therapy correlate with improvements in vertebral strength in men with glucocorticoid-induced osteoporosis. Osteoporos Int 24:2971–2981. doi:10.1007/s00198-013-2379-5
Saag KG, Shane E, Boonen S et al (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Eng J Med 357:2028–2039. doi:10.1056/NEJMoa071408
Harslǿf T, Langdhal BL (2016) New horizons in osteoporosis therapies. Curr Opin Pharmacol 28:38–42
Papapoulos SE, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteopros Int 26(12):2773–2783. doi:10.1007/s00198-015-3234-7
Papapoulos SE, Makras P (2008) Selection of antiresorptive or anabolic treatments for postmenopausal osteoporosis. Nat Clin Pract End Metab 4(9):514–523. doi:10.1038/ncpendmet0941
Adler RA (2016) Osteoporosis treatment: complexities and challenges. J Endocrinol Invest 39(7):719–720. doi:10.1007/s40618-016-0437-5
Miller PD, Hattersley G, Riis BJ et al (2016) Effect of Abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316(7):722–733. doi:10.1001/jama.2016.11136
Johnell O, Kanis JA, Odén A et al (2004) Fracture risk following an osteoporotic fracture. Osteoporos Int 15:175. doi:10.1007/s00198-003-1514-0
Cosman F, Hattersley G, Hu MY et al (2017) Effects of Abaloparatide-SC on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. J Bone Miner Res 32:17–23. doi:10.1002/jbmr.2991
Costa AG, Bilezikian JP, Lewiecki EM (2014) Update on romosozumab: a humanized monoclonal antibody to sclerostin. Expert Opin Biol Ther 14(5):697–707. doi:10.1517/14712598.2014.895808
Drake MT, Srinivasan B, Mödder UI et al (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95(11):5056–5062. doi:10.1210/jc.2010-0720
Piemonte S, Romagnoli E, Bratengeier C et al (2012) Serum sclerostin levels decline in post-menopausal women with osteoporosis following treatment with intermittent parathyroid hormone. J Endocrinol Invest 35:866–868. doi:10.3275/8522
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33(5):747–783. doi:10.1210/er.2011-1060
Cosman F, Crittenden DB, Adachi JD et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Eng J Med 375(16):1532–1543
Genant HK, Engelke K, Bolognese MA et al (2017) Effects of Romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res 32:181–187. doi:10.1002/jbmr.2932
McColm J, Hu L, Womack T, Tang CC, Chiang AY (2014) Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res 29:935–943. doi:10.1002/jbmr.2092
Recknor CP, Recker RR, Benson CT (2015) The effect of discontinuing treatment with Blosozumab: follow-up results of a phase 2 randomized clinical trial in postmenopausal women with low bone mineral density. J Bone Miner Res 30:1717–1725. doi:10.1002/jbmr.2489
Bone HG, Dempster DW, Eisman JA et al (2015) Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the long-term odanacatib fracture trial. Osteoporos Int 26(2):699–712. doi:10.1007/s00198-014-2944-6
Bonafede MM, Shi N, Bower AG et al (2015) Teriparatide treatment patterns in osteoporosis and subsequent fracture events: a US claims analysis. Osteoporos Int 26:1203–1212. doi:10.1007/s00198-014-2971-3
Cosman F, Nieves JW, Dempster DW (2017) Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res 32:198–202. doi:10.1002/jbmr.3051
Cosman F, Wermers RA, Recknor C et al (2009) Effects of Teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab 94(10):3772–3780. doi:10.1210/jc.2008-2719
Boonen S, Marin F, Obermayer-Pietsch B et al (2008) Effects of previous antiresorptive therapy on the bone mineral density response to 2 years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 93(3):852–860. doi:10.1210/jc.2007-0711
Cosman F, Keaveny TM, Kopperdahl D et al (2013) Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J Bone Miner Res 28:1328–1336. doi:10.1002/jbmr.1853
Leder BZ, Tsai JN, Uihlein AV et al (2014) Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (the DATA extension study): a randomized controlled trial. J Clin Endocrinol Metab 99(5):1694–1700. doi:10.1210/jc.2013-4440
Leder BZ, Tsai JN, Uihlein AV et al (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-switch study): extension of a randomised controlled trial. Lancet 386(9999):1147–1155. doi:10.1016/S0140-6736(15)61120-5
Leder BZ, Tsai JN, Jiang LA, Lee H (2017) Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: the Denosumab and Teriparatide follow-up study (DATA-follow-up). Bone 98:54–58. doi:10.1016/j.bone.2017.03.006
Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O (2016) Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 27(5):1923–1925. doi:10.1007/s00198-015-3380-y
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof. S. Minisola served as speaker for Abiogen, Amgen, Bruno Farmaceutici, Diasorin, Eli Lilly and Fujii. He also served on the Advisory Board of Abiogen. He received consultancy from Bruno Farmaceutici.
Statement of human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not required.
Funding
There was no specific funding for this work.
Rights and permissions
About this article
Cite this article
Minisola, S., Cipriani, C., Occhiuto, M. et al. New anabolic therapies for osteoporosis. Intern Emerg Med 12, 915–921 (2017). https://doi.org/10.1007/s11739-017-1719-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-017-1719-4