Abstract
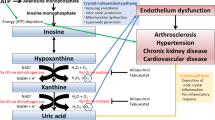
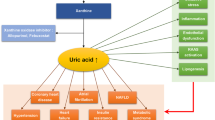
Elevated uric acid levels are a common finding in patients with metabolic syndrome and in those with cardiovascular and renal disease, but the meaning of this elevation is still unclear. In patients with chronic kidney diseases, it could merely reflect the reduction in glomerular filtration rate: but uric acid levels are known to be elevated in people, also in younger ones, prior to the development of hypertension or renal disease, independently of several risk factors. Multiple potential mechanisms suggest a causative role for uric acid in vascular disease. Uric acid has been shown to be involved in metabolic pathways that lead to oxidative stress, endothelial disfunction, and to a vascular and systemic inflammatory response. Moreover, the elevation in uric acid levels observed after fructose ingestion, with a consequent reduction in nitric oxide, may lead to a reduced glucose uptake in the skeletal muscle, hyperinsulinemia, and insulin resistance. Besides these bench research data, also clinical studies showed the beneficial effects of lowering uric acid therapies on several markers of cardiovascular and renal disease. To date, however, there is no evidence indicating that such therapies, that are not free of risk, may reduce cardiovascular events; so that to manage our prescriptions, we need larger, prospective, interventional data.
Similar content being viewed by others
References
World Health Organization (1999) Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. Diabet Med 15(7): 539–553. (Available at http://whqlibdoc.who.int/hq/1999/who_ncd_ncs_99.2.pdf)
National Cholesterol Education Program (2002) Third report of the national cholesterol education program (NCEP) on detection and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 106:3143–3421
Alberti KGM, Zimmet P, Shaw J (2006) Metabolic syndrome: a new world-wide definition. A consensus statement from the International diabetes federation. Diabet Med 23:469–480
Chen J, Muntner P, Hamm L et al (2004) The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med 140:167–174
Wahba IM, Mak RH (2007) Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2:550–562
Ramirez SP, McClellan W, Port FK et al (2002) Risk factors for proteinuria in a large, multiracial, Southeast Asian population. J Am Soc Nephrol 13:1907–1917
Tozawa M, Iseki K, Iseki C et al (2002) Influence of smoking and obesity on the development of proteinuria. Kidney Int 62:956–962
Trayhurn P (2005) Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand 184:285–293
Thatcher S, Yiannikouris F, Gupte M et al (2009) The adipose renin-angiotensin system: role in cardiovascular disease. Mol Cell Endocrinol 302:111–117
Iseki K (2008) Metabolic syndrome and chronic kidney disease: a Japanese perspective on a worldwide problem. J Nephrol 21:305–312
Gertler MM, Garn SM, Levine SA (1951) Serum uric acid in relation to age and physique in health and in coronary heart disease. Ann Intern Med 34:1421–1431
Cannon PJ, Stason WB, Demartini FE et al (1966) Hyperuricemia in primary and renal hypertension. N Engl J Med 275:457–464
Bos MJ, Koudstaal PJ, Hofman A et al (2006) Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam Study. Stroke 37:1503–1507
Feig DI, Kang DH, Nakagawa T et al (2006) Uric acid and hypertension. Curr Hypertens Rep 8:111–115
Obermayr RP, Temml C, Knechtelsdorfer M et al (2008) Predictors of new-onset decline in kidney function in a general middle-european population. Nephrol Dial Transplant 23:1265–1273
Weiner DE, Tighiouart H, Elsayed EF et al (2008) Uric acid and incident kidney disease in the community. J Am Soc Nephrol 19:1204–1211
K/DOQI (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39(S1):S1–S266
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821
Feig DI, Johnson RJ (2003) Hyperuricemia in childhood primary hypertension. Hypertension 42:247–252
Feig DI, Mazzali M, Kang D-H et al (2006) Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol 17:S69–S73
Gertzberg N, Neumann P, Rizzo V et al (2004) NAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-alpha. Am J Physiol Lung Cell Mol Physiol 286:L37–L48
Sautin YY, Nakagawa T, Zharikov S et al (2007) Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol 293:C584–C596
Mazzali M, Hughes J, Kim YG et al (2001) Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38:1101–1106
Sarafidis PA, Bakris GL (2007) Insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab 92:379–385
TerMaaten JC, Voorburg A, Heine RJ et al (1997) Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci (Lond) 92:51–58
Heinig M, Johnson RJ (2006) Role of uric acid in hypertension, renal disease, and metabolic syndrome. Cleve Clin J Med 73:1059–1064
Hallfrisch J (1990) Metabolic effects of dietary fructose. FASEB J 4:2652–2660
Viazzi F, Leoncini G, Vercelli M et al (2011) Serum uric acid levels predict new-onset type 2 diabetes in hospitalized patiants with primary hypertension: the MAGIC study. Diabetes Care 34:126–128
Nakagawa T, Cirillo P, Sato W et al (2009) The conundrum of hyperuricemia, metabolic syndrome, and renal disease. Intern Emerg Med 3:313–318
Mazzali M, Kanellis J, Han L et al (2002) Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 282:F991–F997
Ames BN, Cathcart R, Schwiers E et al (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 78:6858–6862
Sautin YY, Johnson RJ (2008) Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 27:608–619
So A, Thorens B (2010) Uric acid transport and disease. J Clin Invest 120:1791–1799
Higgins P, Dawson J, Walters M (2009) The potential for xanthine oxidase inhibition in the prevention and treatment of cardiovascular and cerebrovascular disease. Cardiovasc Psychiatry Neurol 2009:282059
Noman A, Ang DSC, Ogston S et al (2010) Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomised, placebo controlled crossover trial. Lancet 9732:2161–2167
Goicoechea M, Vinuesa SG, Verdalles U et al (2010) Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 5(8):1388–1393
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stellato, D., Morrone, L.F., Di Giorgio, C. et al. Uric acid: a starring role in the intricate scenario of metabolic syndrome with cardio-renal damage?. Intern Emerg Med 7, 5–8 (2012). https://doi.org/10.1007/s11739-011-0642-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-011-0642-3