Abstract
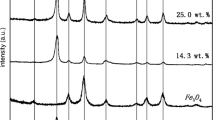
Multi-walled carbon nanotubes (MWCNTs) can act not only as a support for Fe3O4 nanoparticles (NPs) but also as a coworker with synergistic effect, accordingly improving the heterogeneous Fenton-like efficiency of Fe3O4 NPs. In this study, Fe3O4 NPs were in situ anchored onto MWCNTs by a moderate co-precipitation method and the as-prepared Fe3O4/MWCNTs nanocomposites were employed as the highly efficient Fenton-like catalysts. The analyses of XRD, FTIR, Raman, FESEM, TEM and HRTEM results indicated the formation of Fe3O4 crystals in Fe3O4/MWCNTs nanocomposites prepared at different conditions and the interaction between Fe3O4 NPs and MWCNTs. Over a wide pH range, the surface of modified MWCNTs possessed negative charges. Based on these results, the possible combination mechanism between Fe3O4 NPs and MWCNTs was discussed and proposed. Moreover, the effects of preparation and catalytic conditions on the Fenton-like catalytic efficiency were investigated in order to gain further insight into the heterogeneous Fenton-like reaction catalyzed by Fe3O4/MWCNTs nanocomposites.
Similar content being viewed by others
References
Cai Z Q, Sun Y M, Liu W, et al. An overview of nanomaterials applied for removing dyes from wastewater. Environmental Science and Pollution Research International, 2017, 24(19): 15882–15904
Freyria F S, Armandi M, Compagnoni M, et al. Catalytic and photocatalytic processes for the abatement of N-containing pollutants from wastewater. Part 2: organic pollutants. Journal of Nanoscience and Nanotechnology, 2017, 17(6): 3654–3672
Holkar C R, Jadhav A J, Pinjari D V, et al. A critical review on textile wastewater treatments: possible approaches. Journal of Environmental Management, 2016, 182: 351–366
Quadrado R F N, Fajardo A R. Fast decolorization of azo methyl orange via heterogeneous Fenton and Fenton-like reactions using alginate-Fe2+/Fe3+ films as catalysts. Carbohydrate Polymers, 2017, 177: 443–450
Feng J X, Li S Y, Sheng Y, et al. Remarkable improvement of cycling Fenton process for catalytic degradation of phenol: tuning of triggering effect. Applied Catalysis A: General, 2017, 542: 21–27
Clarizia L, Russo D, Somma D I, et al. Homogeneous photo-Fenton processes at near neutral pH: a review. Applied Catalysis B: Environmental, 2017, 209: 358–371
Mirzaei A, Chen Z, Haghighat F, et al. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes: a review. Chemosphere, 2017, 174: 665–688
Teixeira A P C, Tristão J C, Araujo M H, et al. Iron: a versatile element to produce materials for environmental applications. Journal of the Brazilian Chemical Society, 2012, 23(9): 1579–1593
Yang S, Wu P, Yang Q, et al. Regeneration of iron-montmorillonite adsorbent as an efficient heterogeneous Fenton catalytic for degradation of Bisphenol A: structure, performance and mechanism. Chemical Engineering Journal, 2017, 328: 737–747
Abass O K, Zhuo M S, Zhang K S. Concomitant degradation of complex organics and metals recovery from fracking wastewater: roles of nanozerovalent iron initiated oxidation and adsorption. Chemical Engineering Journal, 2017, 328: 159–171
Zhong Y H, Yu L, Chen Z F, et al. Microwave-assisted synthesis of Fe3O4 nanocrystals with predominantly exposed facets and their heterogeneous UVA/Fenton catalytic activity. ACS Applied Materials & Interfaces, 2017, 9(34): 29203–29212
Liu Y Y, Jin W, Zhao Y P, et al. Enhanced catalytic degradation of methylene blue by a-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions. Applied Catalysis B: Environmental, 2017, 206: 642–652
Wang Y, Fang J S, Crittenden J C, et al. Novel RGO/a-FeOOH supported catalyst for Fenton oxidation of phenol at a wide pH range using solar-light-driven irradiation. Journal of Hazardous Materials, 2017, 329: 321–329
Xiao F, Li W T, Fang L P, et al. Synthesis of akageneite (β-FeOOH)/reduced graphene oxide nanocomposites for oxidative decomposition of 2-chlorophenol by Fenton-like reaction. Journal of Hazardous Materials, 2016, 308: 11–20
Xu L J, Wang J L. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Applied Catalysis B: Environmental, 2012, 123–124: 117–126
Rusevova K, Kopinke F D, Georgi A. Nano-sized magnetic iron oxides as catalysts for heterogeneous Fenton-like reactions: influence of Fe(II)/Fe(III) ratio on catalytic performance. Journal of Hazardous Materials, 2012, 241–242: 433–440
Li K Y, Zhao Y Q, Song C S, et al. Magnetic ordered mesoporous Fe3O4/CeO2 composites with synergy of adsorption and Fenton catalysis. Applied Surface Science, 2017, 425: 526–534
Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proceedings of the Royal Society, 1934, 147(861): 332–351
Ribeiro R S, Silva A M T, Tavares P B, et al. Hybrid magnetic graphitic nanocomposites for catalytic wet peroxide oxidation applications. Catalysis Today, 2017, 280: 184–191
Tian X, Jin H, Nie Y, et al. Heterogeneous Fenton-like degradation of ofloxacin over a wide pH range of 3.6–10.0 over modified mesoporous iron oxide. Chemical Engineering Journal, 2017, 328: 397–405
Tang X K, Feng Q M, Liu K, et al. Fabrication of magnetic Fe3O4/ silica nanofiber composites with enhanced Fenton-like catalytic performance for Rhodamine B degradation. Journal of Materials Science, 2018, 53(1): 369–384
Zhou Z Y, Su M H, Shih K M. Highly efficient and recyclable graphene oxide-magnetite composites for isatin mineralization. Journal of Alloys and Compounds, 2017, 725: 302–309
Xu H Y, Shi T N, Zhao H, et al. Heterogeneous Fenton-like discoloration of methyl orange using Fe3O4/MWCNTs as catalyst: process optimization by response surface methodology. Frontiers of Materials Science, 2016, 10(1): 45–55
Jafari A J, Kakavandi B, Jaafarzadeh N, et al. Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: adsorption and degradation studies. Journal of Industrial and Engineering Chemistry, 2017, 45: 323–333
Zhao H, Weng L, Cui W W, et al. In situ anchor of magnetic Fe3O4 nanoparticles onto natural maifanite as efficient heterogeneous Fenton-like catalyst. Frontiers of Materials Science, 2016, 10(3): 300–309
Wan D, Wang G H, Li W B, et al. Investigation into the morphology and structure of magnetic bentonite nanocomposites with their catalytic activity. Applied Surface Science, 2017, 413: 398–407
Ribeiro R S, Silva A M T, Figueiredo J L, et al. Catalytic wet peroxide oxidation: a route towards the application of hybrid magnetic carbon nanocomposites for the degradation of organic pollutants. A review. Applied Catalysis B: Environmental, 2016, 187: 428–460
Deng J H, Wen X H, Wang Q N. Solvothermal in situ synthesis of Fe3O4–multiwalled carbon nanotubes with enhanced heterogeneous Fenton-like activity. Materials Research Bulletin, 2012, 47 (11): 3369–3376
Tian X, Liu Y, Chi W, et al. Catalytic degradation of phenol and pnitrophenol using Fe3O4/MWCNT nanocomposites as heterogeneous Fenton-like catalyst. Water, Air, and Soil Pollution, 2017, 228(8): 297
Aboutalebi S H, Chidembo A T, Salari M, et al. Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy & Environmental Science, 2011, 4(5): 1855–1865
Hu X B, Liu B Z, Deng Y H, et al. Adsorption and heterogeneous Fenton degradation of 17a-methyltestosterone on nano Fe3O4/ MWCNTs in aqueous solution. Applied Catalysis B: Environmental, 2011, 107(3–4): 274–283
Xu H Y, Zheng Z, Mao G J. Enhanced photocatalytic discoloration of acid fuchsine wastewater by TiO2/schorl composite catalyst. Journal of Hazardous Materials, 2010, 175 (1–3): 658–665
Zubir N A, Motuzas J, Yacou C, et al. Graphene oxide with zinc partially substituted magnetite (GO-Fe1–xZnxOy) for the UVassisted heterogeneous Fenton-like reaction. RSC Advances, 2016, 6(50): 44749–44757
Wang X, Zhao Z, Qu J, et al. Fabrication and characterization of magnetic Fe3O4–CNT composites. Journal of Physics and Chemistry of Solids, 2010, 71(4): 673–676
Wang H, Jiang H, Wang S, et al. Fe3O4–MWCNT magnetic nanocomposites as efficient peroxidase mimic catalysts in a Fenton-like reaction for water purification without pH limitation. RSC Advances, 2014, 4(86): 45809–45815
Stobinski L, Lesiak B, Kövér L, et al. Multiwall carbon nanotubes purification and oxidation by nitric acid studied by the FTIR and electron spectroscopy methods. Journal of Alloys and Compounds, 2010, 501(1): 77–84
Song S, Rao R, Yang H, et al. Facile synthesis of Fe3O4/MWCNTs by spontaneous redox and their catalytic performance. Nanotechnology, 2010, 21(18): 185602
Yu L, Yang X, Ye Y, et al. Efficient removal of atrazine in water with a Fe3O4/MWCNTs nanocomposite as a heterogeneous Fenton-like catalyst. RSC Advances, 2015, 5(57): 46059–46066
Shamsudin M S, Asli N A, Abdullah S, et al. Effect of synthesis temperature on the growth iron-filled carbon nanotubes as evidenced by structural, micro-Raman, and thermogravimetric analyses. Advances in Condensed Matter Physics, 2012, 420619 (7 pages)
Chen G, Futaba D N, Sakurai S, et al. Interplay of wall number and diameter on the electrical conductivity of carbon nanotube thin films. Carbon, 2014, 67: 318–325
Futaba D N, Yamada T, Kobashi K, et al. Macroscopic wall number analysis of single-walled, double-walled, and few-walled carbon nanotubes by X-ray diffraction. Journal of the American Chemical Society, 2011, 133(15): 5716–5719
Kim B, Sigmund W M. Functionalized multiwall carbon nanotube/gold nanoparticle composites. Langmuir, 2004, 20 (19): 8239–8242
Li D, Müller M B, Gilje S, et al. Processable aqueous dispersions of graphene nanosheets. Nature Nanotechnology, 2008, 3(2): 101–105
Iida H, Takayanagi K, Nakanishi T, et al. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. Journal of Colloid and Interface Science, 2007, 314(1): 274–280
Cheng Z P, Chu X Z, Yin J Z, et al. Surfactantless synthesis of Fe3O4 magnetic nanobelts by a simple hydrothermal process. Materials Letters, 2012, 75: 172–174
Zhang J, Liu G D, Wang P H, et al. Facile synthesis of FeOCl/iron hydroxide hybrid nanosheets: enhanced catalytic activity as a Fenton-like catalyst. New Journal of Chemistry, 2017, 41(18): 10339–10346
Hassan H, Hameed B H. Fe-clay as effective heterogeneous Fenton catalyst for the decolorization of Reactive Blue 4. Chemical Engineering Journal, 2011, 171(3): 912–918
Xu H Y, Prasad M, Liu Y. Schorl: a novel catalyst in mineralcatalyzed Fenton-like system for dyeing wastewater discoloration. Journal of Hazardous Materials, 2009, 165(1–3): 1186–1192
Kwan W P, Voelker B M. Rates of hydroxyl radical generation and organic compound oxidation in mineral-catalyzed Fenton-like systems. Environmental Science & Technology, 2003, 37(6): 1150–1158
Feng J, Hu X, Yue P L. Novel bentonite clay-based Fenanocomposite as a heterogeneous catalyst for photo-Fenton discoloration and mineralization of Orange II. Environmental Science & Technology, 2004, 38(1): 269–275
Acknowledgement
This work was financially supported by the Natural Science Foundation of Heilongjiang Province, China (No. E2015065).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, HY., Wang, Y., Shi, TN. et al. Heterogeneous Fenton-like discoloration of methyl orange using Fe3O4/MWCNTs as catalyst: combination mechanism and affecting parameters. Front. Mater. Sci. 12, 21–33 (2018). https://doi.org/10.1007/s11706-018-0408-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-018-0408-1